Pfizer is voluntarily recalling Accuretic (quinapril HCl/hydrochlorothiazide) tablets distributed by Pfizer as well as two authorized generics distributed by Greenstone (quinapril and hydrochlorothiazide and quinapril HCl/ hydrochlorothiazide) to the patient (consumer/user) level due to the presence of a nitrosamine, N-nitroso-quinapril, above the Acceptable Daily Intake (ADI) level.
Pfizer will recall six lots of Accuretic tablets, one lot of quinapril and hydrochlorothiazide tablets and four lots of quinapril HCl/ hydrochlorothiazide tablets.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/pfizer-voluntary-nationwide-recall-lots-accuretictm-quinapril-hclhydrochlorothiazide-quinapril-and?utm_medium=email&utm_source=govdelivery
2 Likes
For awareness this appears to have triggered requests for information from Swiss Medic relating to all ACE inhibitors. this has included very short deadlines for responses -25th March
3 Likes
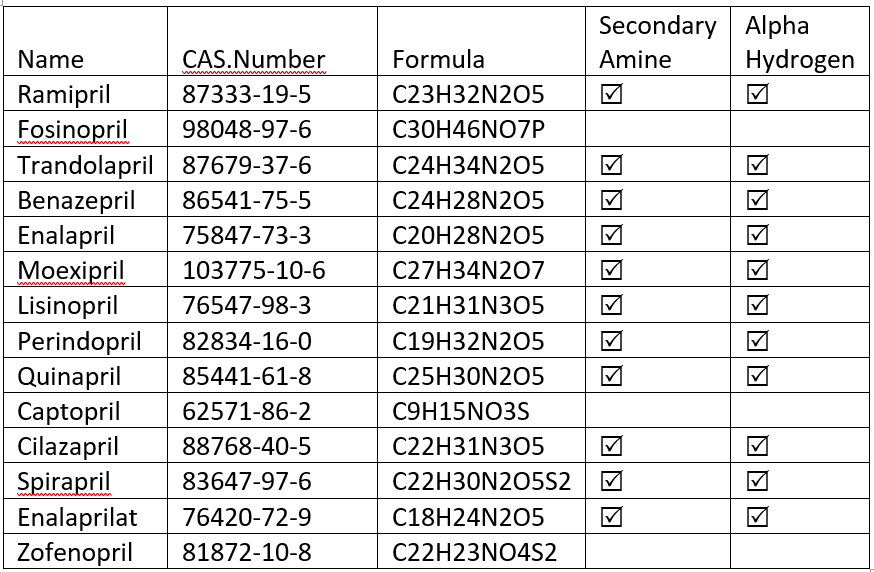
This is the list of ACE inhibitors I was able to pull from GSRS. I have flagged secondary amines and Alpha Hydrogen in the structure.
5 Likes
Hey Naiffer,
Where did you find these data? I am currently looking for a pharmaceutical sample, which possible containes NDMA, NDEA or NDBA, and I would like to do the same risk analyses as you did.
Thanks for the help, in advance!
@Tom the data above was an assessment of ACE inhibitors from the GSRS (GSRS). Flagging presence of secondary amines and alpha hydrogen was done manually
@Naiffer_Host Ah okay, thanks!