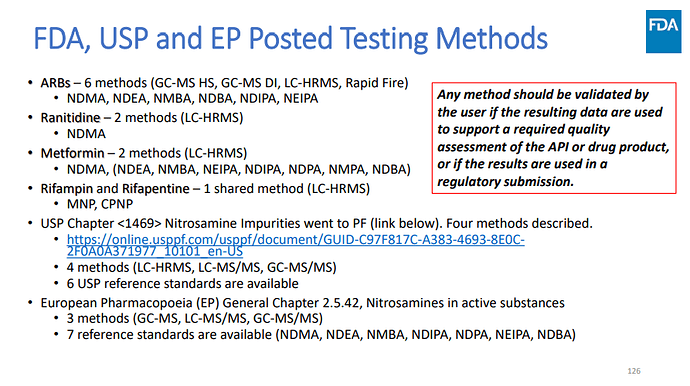
At a recent workshop on Nitrosamine Impurities, Dr. David Keire (CDER-FDA) shared a list of referential testing methods. It was also clearly stated that “any method should be validated by the user if the resulting data are used to support a required quality assessment of the API or drug product, or if the results are used in a regulatory submission”.
- What testing methods is your team/organization using?
- FDA/USP/EP Methods
- In-house developed Methods
- Instrument manufacture Methods
- API/Excipient/DP supplier Methods
0 voters