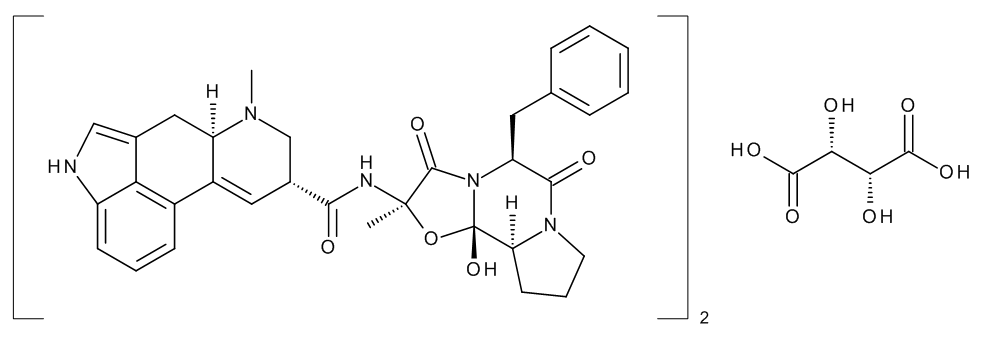
I’m working on a risk analysis for a product containing Caffeine and Ergotamine Tartrate. Do you think there is any risk related to the API Ergotamine Tartrate regarding the formation of nitrosamines, given that the molecule features one basic tertiary amino group, a pyrrole ring and an amide group?
Yes. I would be particularly concerned for the amine group on the far left of the structure.
I would suggest you to perform a search on this site for “indole”. There were a few documented discussions on the possibility of nitrosation and AI levels of compounds including indole structure.
Of course, the risk assessment will not be limited only to the structure of this molecule, but include a comprehensive analysis of all aspects of API and drug product manufacturing, impurities, stability, etc.
There is another N which is part of a amide of a secondary amine, which could be an issue, too.
As suggested, I would look for related impurities. The amine on the far left is, as mentioned, an indole-like amine, which is outside of the scope of CPCA. As mentioned, is best to look a bit more in this forum.
Regarding the amides mentioned, to be honest I would not worry at first, but it may be best to at least try and test the reactivity, and the tertiary amides that are part of 5- and 6-member rings, hydrolysis is not going to be easy, so I would not expect any issues.
Having said that, a thorough evaluation of related substances should be carried out (is there any open-ring impurity that leaves a secondary amine available?
The one that would worry me the most is the tertiary amine that contains the methyl group. I would not be very surprised if one of the related substances was the desmethyl derivative, and, if so, a very reactive precursor is present.