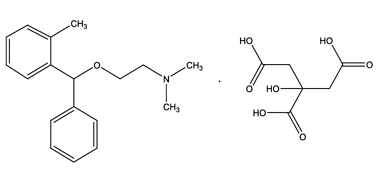
Sandoz Inc. is initiating a voluntary recall of 13 lots of oral Orphenadrine citrate 100 mg Extended Release (ER) Tablets to the consumer level. The presence of a nitrosamine (N-methyl-N-nitroso-2-[(2-methylphenyl)phenylmethoxy]ethanamine (NMOA or Nitroso-Orphenadrine)) impurity, which has the potential to be above the U.S. Food and Drug Administration (FDA)’s Acceptable Daily Intake (ADI) limit of 26.5 ng/day, was detected in the lots during recent testing. These 13 lots of Orphenadrine Citrate ER Tablets were shipped to customers from August 2019 to April 2021.
There are 2 potential routes for formation of this impurity. One is via the tertiary amine and dialkylative N-Nitrosation - this in the solid phase is unlikely, unless this occurs in the API synthesis.
Another route would be if there was a secondary amine (des methyl) impurity in the API that is nitrosated in the formulation either during formulation or on storage
Thanks Andy, Achana!
@Samo @vesna @Manja @Kaja @fdebock any insight on the findings, have you confirmed the formation path of the impurity?
@AndyTeasdale I had the opportunity this weekend to go over the compendial impurities for Orphenadrine Citrate Monograph and ER product.
Orphenadrine Citrate
Related compound A
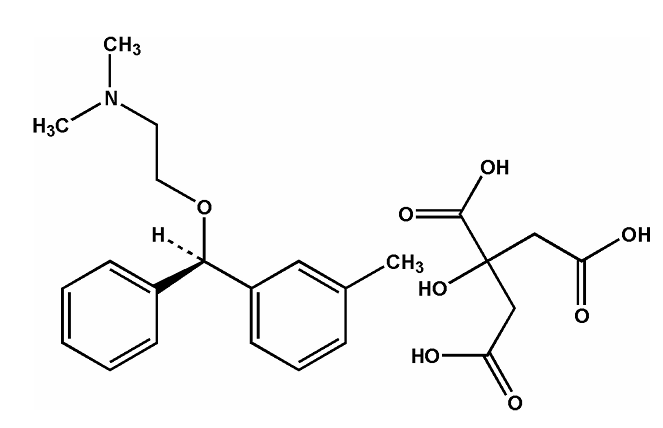
Related compound B
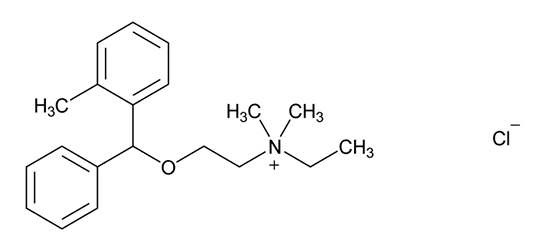
Related compound C - USP [NMT 0.5%]
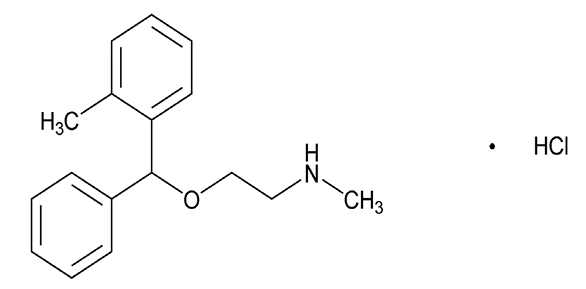
Related compound E
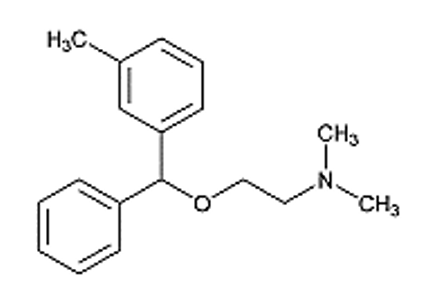
Related compound F
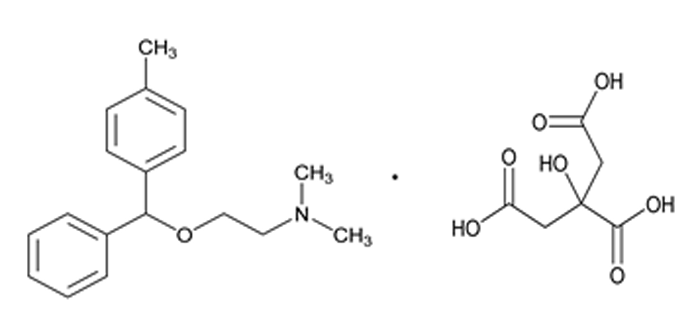
 @schlinjo1975 do we need to expand the search to API impurities as well?
@schlinjo1975 do we need to expand the search to API impurities as well?
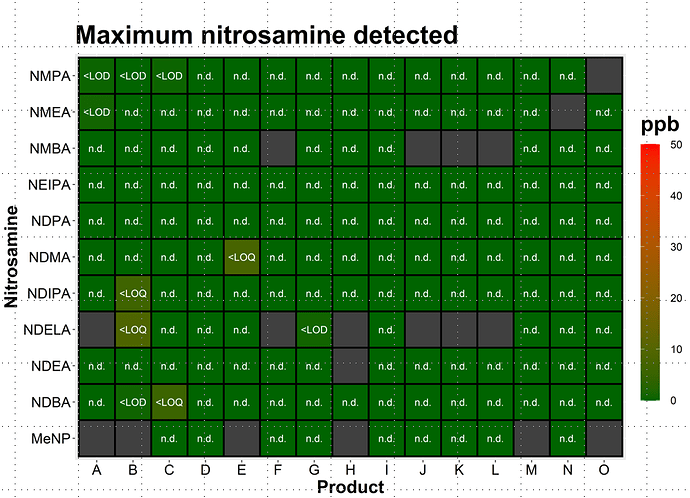
Yes, I do think so. In principle, any vulnerable amine could potentially be nitrosated. Such vulnerable amines could be small secondary amines like DMA or DEA, but it could also be the API or API impurities. The risk from secondary amine API impurities is lower compared to the risk from secondary amine APIs itself due to the much smaller amount in the product. However, due to the low default AI of 18 ng/day and the resulting nitrosamine limits in the ppb range they definitely need to be considered. So far, we have tested for one API impurity derived nitrosamine and have detected it.
@schlinjo1975 We can consider a secondary/tertiary/quarternairy amine as potential precursor for a nitrosamine to be nitrosated, when under the right conditions. Do you have any view on the possible presence of NDBA, NDEA, or NDMA in medicines, which are still on the market?
As part of our confirmatory testing exercise we have screened applicalble products for the presence of 11 “standard nitrosamines”, i.e. small nitrosamines such as NDMA, NDEA, NMBA etc. and found… essentially nothing. But every product is different, so this doesn’t mean that all products on the market are free from these small nitrosamines. I think the general risk is rather low, though, much lower than the risk of seeing complex, API-derived nitrosamines.
Hey @schlinjo1975, thanks for your reply! This research seems really interesting to me! Is there any chance that you could share which products you’ve analysed (or at least define why you specifically chose the products)? (f.e. on the presence of secundairy/tertiary amines in either the API, the drug itself, or in the reactants).
I am developing a method for the determination of NDMA, NDEA, NDBA, NPIP, NPYR, and NMEA. To identify if this method really works, I want to analyse a sample containing at least one of these NAs. It’s really difficult to obtain such a sample from drug suppliers itself. Also it’s really hard to identify such a drug using the literature.
Do you have any recommendations? It would be much appreaciated!
The results on the analysis of eleven nitrosamines in the pharmaceutical products is so interesting to me.
I am now preparing a standard mixture of 6 nitrosamines listed in the <1469> Nitrosamine Impurities. I rather think to change the standard mix list to NMPA, NMEA, NDMA, NDIPA, NDELA and NDBA.