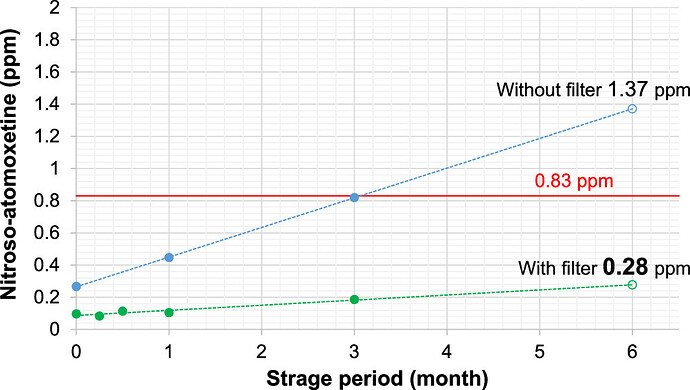
The Towa Pharmaceutical Team in Japan published a new paper. They successfully reduced the amount of Nitroso-atomoxetine to an acceptable level (NMT 0.83 ppm) with an NOx removal filter. It’s a fantastic approach!
https://pubs.acs.org/doi/10.1021/acs.oprd.5c00182
The three-pronged approach means the following requirements for APIs, excipients, and air.
a) APIs: Selection of APIs where nitroso-atomoxetine, vulnerable secondary amine impurities, and low molecular weight nitrosamine impurities such as NDMA have been removed. Verification of ingredients, intermediates, production processes, solvents for reactions and recrystallization, cross-contamination, storage conditions, and milling.
b) Excipients: Selection of various excipients that had the minimal nitrite content. Verification of the nitrite content and storage conditions.
c) Air: Manufacturing of APIs and drug products under NOx atmospheric conditions at ppb levels, as learned from the metformin research case study. (5) Verification of all processes exposed to air that confirmed the presence or absence of nitrosamine formation.