Evaluating the genotoxicity of nitrosamine impurities and NDSRIs using human TK6 cells transduced with human cytochrome P450s is a research originating from Xilin Li and others indeed (over the years presented on different occassions), but the cell and gene technology behind it is not fully new and should be easily reproducible by labs offering such services.
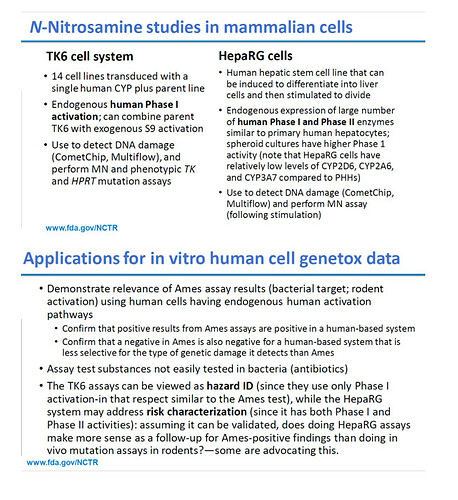
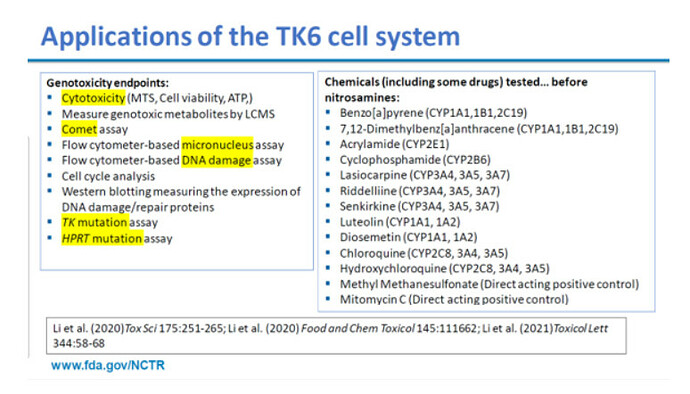
Human lymphoblastoid TK6 cells are amongst the most commonly used mammalian cell lines for in vitro testing (shown by surveys), because they can be grown reliably and easily. Downside is that you need to use exogenous activation for indirect actors because these cell lines do not express cytochrome P450 enzymes. Xilin Li et al. are still using such systems of cell lines with S9 metabolic activation as well, but indeed have on top of this their work published first time in 2020, where they developed a CYP-expressing TK6 cell system for assessing indirect mutagens (having some benefits on representativeness over the use of exogenous activation). In their paper you can find the protocol developed, it was also used for other mutagens than nitrosamines:
Development and Application of TK6-derived Cells Expressing Human Cytochrome P450s for Genotoxicity Testing | Toxicological Sciences | Oxford Academic (oup.com)
Their further contributions:
Evaluation of pyrrolizidine alkaloid-induced genotoxicity using metabolically competent TK6 cell lines - ScienceDirect
The expression of Phase II drug-metabolizing enzymes in human B-lymphoblastoid TK6 cells - PMC (nih.gov)
The genotoxicity potential of luteolin is enhanced by CYP1A1 and CYP1A2 in human lymphoblastoid TK6 cells - ScienceDirect
Genotoxicity evaluation of nitrosamine impurities using human TK6 cells transduced with cytochrome P450s | Archives of Toxicology (springer.com)
In these papers the rationale behind the technique is also explained. You can of course use slightly different protocols, in the end you anyway always proof that the CYP induction has succeeded when you go on with using the cell lines.
The general technique is overall not new, it is based on the commonly used technology to use cDNA vectors for expression of DNA inserts in mammalian cells (existing for decades), also for CYP-expression others have used it before.
With an expression vector containing the genetic information for a targeted CYP enzyme, you introduce this code in the human cells. Once the CYP genes have been inserted, the related CYP protein has to become expressed, which can be checked by qPCR in extracted RNA, allowing to also conform the link with the specific CYP enzyme and confirm successful introduction for the targeted enzyme. The resulting cell lines are called transduced or CYP-expressed TK6 cells (you would have a specific line for each targeted CYP enzyme) and can be used in in vitro mutagenicity test batteries, not only allowing to evaluate micronuclei with the wild type cells, but also the cells induced per enzyme (thus to identify relevant CYP enzymes in case the test item is an indirect mutagen).
For these and other cell lines such CYP expression vectors have also been used by others before, so the Xilin Li et al. approach is not per se unique, but to the best of my knowledge it remains the approach with most examples of application linked to nitrosamines (in vitro mammalian cell gene mutation assay with CYP induced TK6 cells). Considering the Xilin Li 2023 paper and the extra examples presented during SOT2024, this protocol has the advantage of being used already several times on NDSRIs (next to some standard mutagens), but I would not say it has completely replaced the use of exogenous activation or that the protocol discussions has been closed.
In their work focused on NDSRIs, Xilin Li and others use the TK and HPRT cell gene mutation test and the micronucleus test. They do the micronucleus test with S9/uninduced cells, without S9/uninduced cells and without S9/induced cells, sometimes it is not fully clear if with S9/induced cells is also applied. This is probably mostly for comparative and science evolution purposes at the moment, as ICH M7 does not recommend the use of the micronucleus test for indirect acting mutagens. Also the in vitro mammalian gene mutation assays they perform both with S9 induced TK6 cells and CYP-expressing TK6 cell systems.
In addition to this, Xilin Li and others have also published on “metabolically competent” (cf. mRNA expressions checks) 2D and 3D HepaRG cell models, which are undergoing further validation: Genotoxicity assessment of eight nitrosamines using 2D and 3D HepaRG cell models | Archives of Toxicology (springer.com)
Evaluating the mutagenicity of N-nitrosodimethylamine in 2D and 3D HepaRG cell cultures using error-corrected next generation sequencing | Archives of Toxicology (springer.com)
There is still a lot of activity globally on identifying in vitro assays as Ames test follow-up and this is also why this type of research was initially done. In 2024 we say a lot of posters, but a limited number of new papers. I hope to see Mutamind and FDA publications in 2024 providing more details on the more recent studies, especially with new in vivo mutagenicity data appearing in literature on NDSRIs allowing to further evaluate the representativeness of positive in vitro assays in so far these are linkable with low acceptable intakes. It will be a matter of (continuing) comparing relative potency ranking based on different in vitro, in silico and in vivo testing and see if there is a match in the trend across methods. With bits and pieces of information on case studies, it is sometimes difficult to understand if the “nitrosamine TTC” will just shift from 1500 ng/day to 5000 ng/day, possibly with a MW treshold (cf. new publications in vivo mutagenicity studies and potency ranking, e.g; NTTP and N-nitroso-fluoxetine) or if more doubts will be raised on some of the in vitro assays.
Nonetheless, FDA scientists did present in the past CYP-expressed TK6 cell systems as a versatile test system (for nitrosamines and other applications), for example also useful to have a nitrosamine metabolized in vitro with this system and then just analyse the nature of the metabolite analytically (toxifying or detoxifying based on identified structure?). That there are no binding in vitro follow-up tests also gives freedom to design an appropriate justified case-specific WoE approach, although I believe the in vivo testing is often seen as an easier step (as industry is more publishing on in vivo data than in vitro data other than Ames I believe).