CEP申请,EDQM缺陷函内容包括:You are additionally advised that there are several secondary amines/amides in the process (starting materials, intermediates and the API itself) and that active carbon has been recognised as a potential source of nitrite, so this must also be acknowledged in the risk assessment.如何整改?
目前考虑采取以下措施:1、比较不同来源的活性炭,测试其亚硝酸盐(氧化氮)含量;2、根据权威文献中的模型估算反应率和清除效率(最好自己作模式)估算残留的可能;3、如果残留风险较高(高于限度的10%),测试亚硝胺相关杂质。如果残留概率非常低,则不用测试。
The activated carbon scenario has different dimensions:
- Treatment of activated carbon with nitric acid to remove impurities or for changing properties
- Activated carbon as a “catalyst” for nitrosamine formation, even at higher pH and in absence of nitric acid pretreatment (when FDA experts are referring in presentations to catalyst role of activated carbon, they are also citing the Padhye papers in my experience; see also “Activated charcoal surface catalysed formation of nitroso compounds” in Horne 2023). Redirecting
- Retention of some nitrosamines on activated carbon
What is most important, depends on the specific risk assessment.
When a (secondary) amine can have an important interaction with activated carbon particles (absorb to react, even under non-acidic conditions), this can entail a risk for nitrosamine formation based on the studies of Padhye et al:
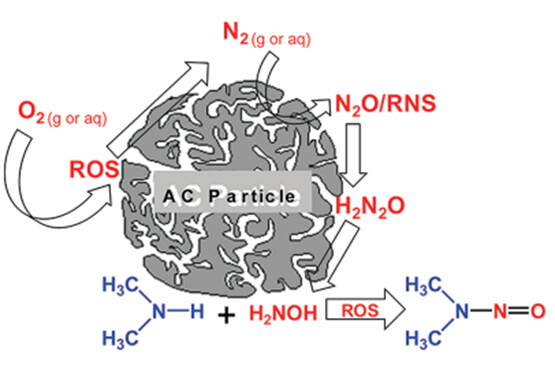
(Image courtesy of Padhye et al. 2011).
This scenario cannot be derisked with nitrite testing on activated carbon.
Padhye, L. P., Hertzberg, B., Yushin, G., & Huang, C.-H. (2011). N-Nitrosamines Formation from Secondary Amines by Nitrogen Fixation on the Surface of Activated Carbon. Environmental Science & Technology, 45(19), 8368–8376. https://doi.org/10.1021/es201696e.
Padhye, L., Wang, P., Karanfil, T., & Huang, C.-H. (2010). Unexpected Role of Activated Carbon in Promoting Transformation of Secondary Amines to N-Nitrosamines. Environmental Science & Technology, 44(11), 4161–4168. https://doi.org/10.1021/es903916t.
See also:
谢谢分享,看来问题更为复杂。除《 药品中亚硝胺杂质的根本原因和风险因素的监管经验》外,其他论文我还没有下载权限,得想办法下载学习学习啦。