The activated carbon scenario has different dimensions:
- Treatment of activated carbon with nitric acid to remove impurities or for changing properties
- Activated carbon as a “catalyst” for nitrosamine formation, even at higher pH and in absence of nitric acid pretreatment (when FDA experts are referring in presentations to catalyst role of activated carbon, they are also citing the Padhye papers in my experience; see also “Activated charcoal surface catalysed formation of nitroso compounds” in Horne 2023). Redirecting
- Retention of some nitrosamines on activated carbon
What is most important, depends on the specific risk assessment.
When a (secondary) amine can have an important interaction with activated carbon particles (absorb to react, even under non-acidic conditions), this can entail a risk for nitrosamine formation based on the studies of Padhye et al:
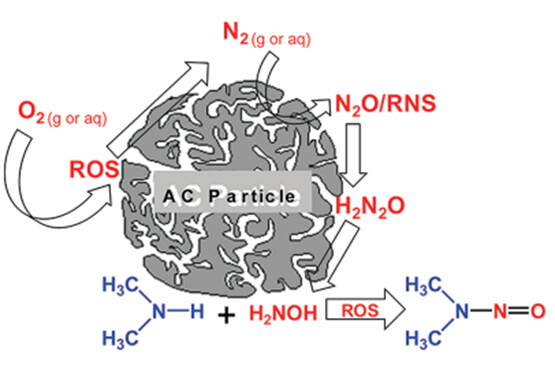
(Image courtesy of Padhye et al. 2011).
This scenario cannot be derisked with nitrite testing on activated carbon.
Padhye, L. P., Hertzberg, B., Yushin, G., & Huang, C.-H. (2011). N-Nitrosamines Formation from Secondary Amines by Nitrogen Fixation on the Surface of Activated Carbon. Environmental Science & Technology, 45(19), 8368–8376. https://doi.org/10.1021/es201696e.
Padhye, L., Wang, P., Karanfil, T., & Huang, C.-H. (2010). Unexpected Role of Activated Carbon in Promoting Transformation of Secondary Amines to N-Nitrosamines. Environmental Science & Technology, 44(11), 4161–4168. https://doi.org/10.1021/es903916t.
See also: