Dear Nitrosamine-Community,
I am currently investigating an analytical method for the NDSRI N-Nitroso Sertraline (monoisotopic mass: 334) using UHPLC-TQ-MS. This method was developed by an external laboratory. However, we observed a significant discrepancy in ion ratios between matrix spike samples and standards prepared in pure solvent, indicating the presence of an interference. All three measured transitions are shared by both the interference and N-Nitroso Sertraline, and no internal standard is being used.
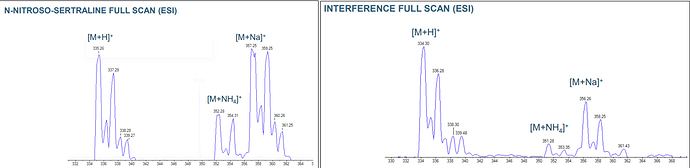
By adjusting the LC parameters, we were able to partially separate the analyte peak from the interference and obtain full-scan spectra for both N-Nitroso Sertraline and the interfering compound (see figure below).
Full-scan analysis revealed that the monoisotopic mass of the interfering compound is 333. Therefore, the observed peaks likely originate from the [M+1] isotope of the interference. Additionally, both N-Nitroso Sertraline and the interfering compound exhibit highly similar isotopic distribution patterns, further supporting the hypothesis that they share the same Sertraline core structure.
It has recently come to my attention that formamide-related impurities can occasionally form from the API. In such cases, the nitroso group (NO, mass: 30) could be replaced by a formamide group (CHO, mass: 29) on the secondary amine of Sertraline. This would be consistent with the observation that the interference has a monoisotopic mass one unit lower than the analyte.
To form a formamide impurity from the API, formic acid is required. While formic acid is absent from the sample preparation process, it is present in the LC solvents (Mobile Phase A: water + 0.1% FA; Mobile Phase B: methanol + 0.1% FA). Given the simplicity of the sample preparation, residual API may remain in the injected sample. My current theory is that, post-injection, residual API could react with formic acid in the LC solvent, generating a formamide impurity that appears as an interference in the chromatogram.
I would be very interested in hearing your thoughts on this hypothesis. Your insights would be greatly appreciated, especially since I will not have access to the TQ for further confirmatory measurements in the near future. My next steps include replacing formic acid with acetic acid and recording an MRM transition using a precursor mass of 334 while keeping the fragment masses unchanged, as well as testing alternative transitions.
Thank you very much, and best regards,
Andreas