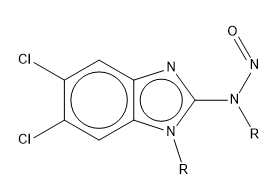
For maribavir, it depends how you define an aromatic bond vs a double bond - the double bond to heteroatom is in an aromatic ring, rather than being a formal double bond, so isn’t perceived as a true double bond in many cheminformatics toolkits, nor does it behave as a guanidine-like structure in this case, which was the intention of the exclusion - to remove the nitrosamide-like structures which can be abiotically hydrolysed to yield diazonium ions from the metabolism-dominated SAR of the CPCA. It can be visualised as the below delocalised form rather than having the explicit double bond: