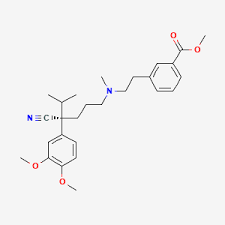
Milestone Pharmaceuticals said on Friday the U.S. health regulator had declined to approve its nasal spray to treat a type of heart condition and had called for an inspection of the facility that performs the testing of the drug.
The company had previously expected to launch the nasal spray, Cardamyst, in mid-2025. The U.S. Food and Drug Administration, in its so-called complete response letter, has also sought additional data on nitrosamine impurities based on new guidance issued after Milestone submitted its application for Cardamyst’s approval. Nitrosamine impurities may increase the risk of cancer if people are exposed to them above acceptable levels and over long periods of time.
Etripamil