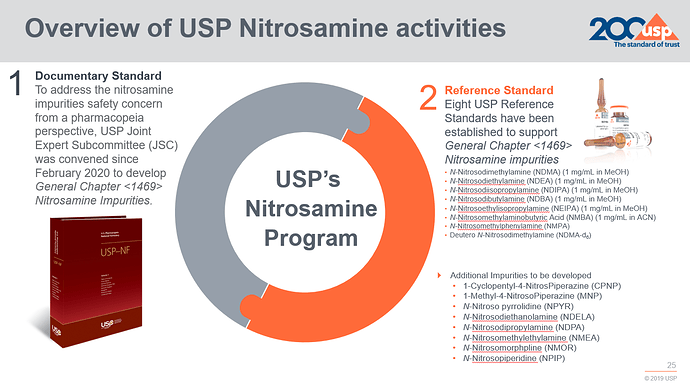
Now USP new General Chapter <1469> Nitrosamines Impurities is available. By providing guidance on assessing for the presence of nitrosamine, establishing control strategies, and monitoring nitrosamine levels in drug products through ensuring the performance of analytical procedures, the new standard supports both manufacturers and regulators, according to the article.
https://www.pharmtech.com/view/usp-s-nitrosamine-impurity-standard-is-now-official
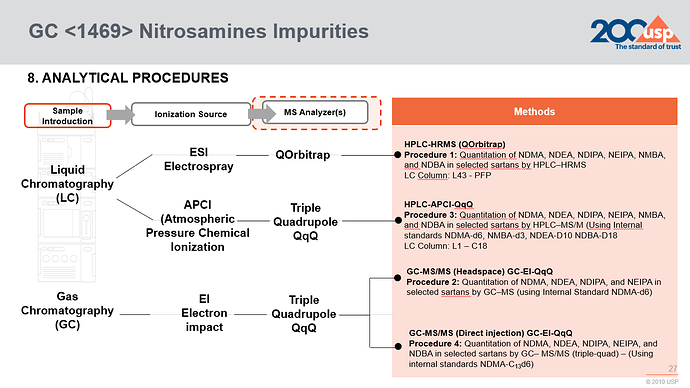
It includes four analytical procedures with LC-HRMS, HSGC-MS, LC-MS/MS, and GC-MS/MS for at most seven nitrosamines and information of seven USP reference standards. The NDMA-d6 RS is not included.