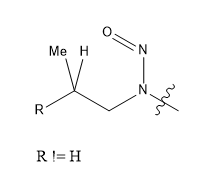
For the “beta-methyl” feature, there is a requirement for the chain to be branched at that position, i.e. n-propyl-like features such as that in ethambutol do not match. In practical terms, beta-methyl is defined essentially along these lines (with an ongoing discussion as to the nature of R; it is definitely not H, OH or Cl, based on the known compounds, it is definitely C, OC (as it would be in beta-methyl nitrosomorpholine), NC (as in beta-methyl nitrosopiperazine), but there might be a few other things):