Category 5
NDSRI list by FDA Category 5.pdf (2.5 MB)
Thanks Yosukemino for valuable inputs!
![]() Thanks ,Very visual!!
Thanks ,Very visual!!
@Yosukemino, this material is excellent! A guide for daily reference!
Fantastic resource @Nitrosamines_Analyzer @trust_user_c @trust_user_a
Nitrosamine Exchange Community ambassador @Yosukemino has built a comprehensive visualization of all NDSRIs listed by the EMA and FDA in their respective revision with their correspondent structures, CPCA score, and suggested AI (ng/da)…
Thanks You Yosuke!
Thanks Yosukemino for valuable contributions.
Thanks a lot, Yosukemino
Good Information, Thank you,
In Guide lines Activating Featurs Aryl Groups (Benzylic or Pseudo Benzylic) they mention shall I consider other than benzylic or pseudo benzylic aryl group for cpca analysis
please give me a reply thank you
My understanding is that the pattern for this is pretty much “any aromatic carbon”, rather than specifically benzyl - so a SMARTS pattern of cCNN=O or similar!
Thank you David thank you for your immediate respond
for Nitrosamine in 6-member ring More than two hetero atoms N, N and O in same ring can we considered deactivating feature score 2 please respond any one thank you
In case I have not mentioned before, please let me say that you are a gem ![]() , Yousukemino San, the visualization helps so much in understaning that CPCA is a systematic approach based on a hypothesis and should be used only where there is no other way to find the AI. If we see the products in Category 1, it is obvious that many of these NOCs do not deserve that label.
, Yousukemino San, the visualization helps so much in understaning that CPCA is a systematic approach based on a hypothesis and should be used only where there is no other way to find the AI. If we see the products in Category 1, it is obvious that many of these NOCs do not deserve that label.
Hi Yosukemino,
Calculation of CPCA category for NDSRIs of Ethambutol.pdf (179.0 KB)
As per FDA, N-nitroso ethambutol has been considered under category-4, as per my point of view methyl group at β-carbon is not considered.
If this is correct then this NDSRI falls under category-3.
I would appreciate your valuable suggestion on this concern.
Detail calculation of CPCA approach is uploaded here.
Thanks.
Thank you for your kind message, @ASrinivasan. I’m glad to see it. And I agree with you. We can perform correct calculations with confidence through case studies. We can mainly focus on the surroundings of N=O. It’s a great system!!
Dear @Amrish_Patel,
I have the same opinion as you. And can someone tell us the answer to this question?
For the “beta-methyl” feature, there is a requirement for the chain to be branched at that position, i.e. n-propyl-like features such as that in ethambutol do not match. In practical terms, beta-methyl is defined essentially along these lines (with an ongoing discussion as to the nature of R; it is definitely not H, OH or Cl, based on the known compounds, it is definitely C, OC (as it would be in beta-methyl nitrosomorpholine), NC (as in beta-methyl nitrosopiperazine), but there might be a few other things):
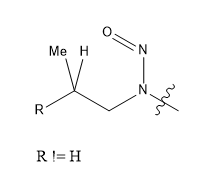
Hi David,
Thanks for your reponse.
If you see the table 4.List of activiting features and associated score in EMA/409815/2020 Rev.17 , page number 33. It is mentioned Methyl group bonded to β-carbon (cyclic or acyclic). Nothing is mentioned about branched methyl group.
Can you please elobrate for me, to get more understanding?
very useful topic …thanks alot
There is a separate topic on this: CPCA - Methyl group bonded to beta carbon (cyclic/acyclic) (activating factor) - Limits of Nitrosamines - Docs - Nitrosamines Exchange (usp.org)
The beta methyl feature is based on theoretic carbocation stabilisation assumption (classic 1,2-akyl or 1,2 H shifts from organic chemistry) and is not per se seen as a consistent trend in carcinogenicity animal studies. As it is truly theoretic it could be a feature that will be debated a lot in case studies and with the development of further readacross science on nitrosamines I believe.
If you want to anyway evaluate to use such considerations to other alkyl chains (for example when evaluating suitability of analogues for readacross or as a justification why out of abundance of caution the activating feature is interpreted in a non-restrictive way), you would have to evaluate the stability and rearrangement possibilities of the specific carbocations, this chemically depends on the nature of the beta alkyl substituent as this determines the possibility for rearrangements and if those rearrangements have a more or less stabilising effect.