Good evening @Yosukemino,
Thanks for your valuable information.
I couldn’t find the N-Nitroso Chlorpromazine limit in the list, Is it possible to post the limit, In calculation it is coming as 100 ng/day AI.
Hi, @kaviraj,
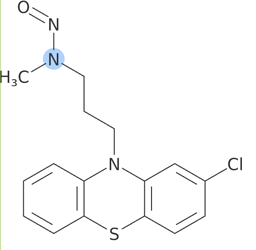
FDA categorizes N-nitroso-desmethyl-chlorpromazine as Category 1 (AI=26.5ng/day).
α-Hydrogen Score(2,3)= +1. And other features are not available. Category 1 looks reasonable.
Thanks for the quick reply.
Thanks for the post. It’s really very helpful.
Can anyone share the structure of the NDSRI that can be formed from Balsalazide and what could be the probable AI? Due to the presence of secondary amine, there is a possibility that NDSRI can be formed but the structure of the probable NDSRI is not reported anywhere.
Thanks in advance.
Hi, @rimita.c,
I could not find the impurity of Balzamide which has secondary amine. If you share the structure of impurity, I may be able to calculate AI with CPCA.
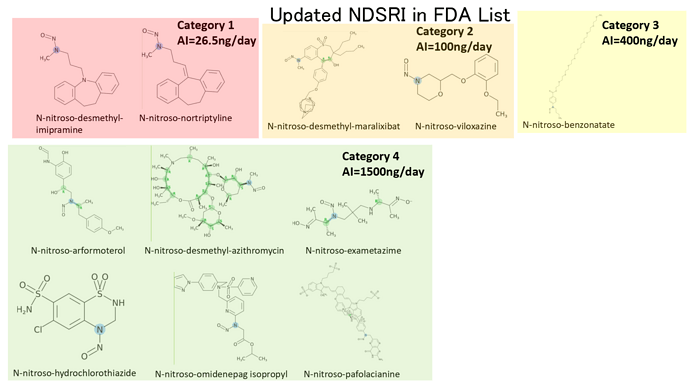
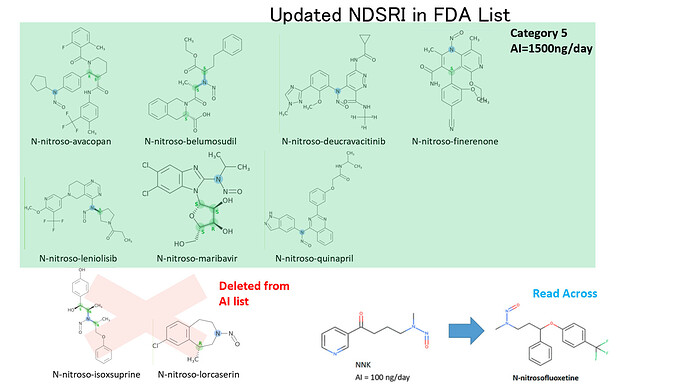
EMA Q&A Appendix 1 was updated. The newly added or updated compounds classified by CPCA are as follows;
Dear Sir,
Limit for Varenicline tartrate by applying CPCA approach it comes under potency category 2.
- Count of hydrogen atoms on each α-carbon (lowest count first) and corresponding α-Hydrogen Score : (2,2) = 1
- Deactivating Feature Score : N-nitroso group in a 7-membered ring= 1
- Activating Feature Score = 0
Potency score = 1 + 1+ 0 = 2
Please correct me if i am wrong.
And EMA published Appendix 1 (28 September 2023
EMA/315970/2023 rev.1) Limit is given 400ng/day.
can you please help me in that.
It’s in both a six and seven membered ring, and the guidance is that the smallest applies - so it gets two points for the ring, for a total of three and an assessment into category 3.
Thank you sir for your valuable reply.
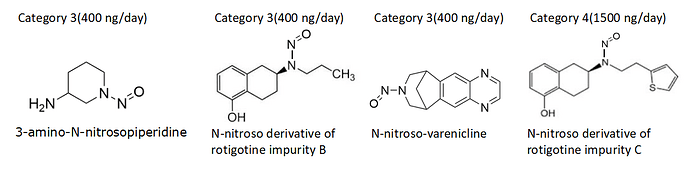
Thank you for explanation! I had the same problem assessing rotigotine impurity B AI as 100 ng based on consideration of propyl chain as methyl group attached to beta-carbon. This specific feature (beta-carbon branched chain) is not really clearly mentioned by EMA in my opinion.
@Yosukemino
In the latest updated of NDSRI from USFDA on 11th Oct 2023 they have shifted N-Nitroso Nortriptyline from Table 2 under read across with limit of 8 ng/day to Table 1 under predicted CPCA wwith limit 26.5ng/day.
What can be the possible justification? Can we find the justification or comments on the official website ? If yes, then please let me know. If no, then kindly throw some light on the probable reason.
Hi, @jasmin.
Thank you for asking. According to my memo, nortriptyline was not included in Table 2 of 1st version. It was likely to be newly classified into Category 1 in Table 1.
And the AI of nortriptyline is 8ng/day in EMA and HC. It is one of the most strict AI in all nitrosamines. But Dr. Nudelman and others mentioned that 8ng/day is not appropriate for nortriptyline and that NNK may be a better surrogate in their fantastic paper Nitrosamine “Saga”.
I have never seen the justification from the FDA, but I guess the FDA wants to make the AI higher than 8ng/day.
This is a great tool and really it is helpful
Hai Dr Yosukemino
Thanks for updating on nitrosamine impurities.
I have a question as below:
To calculate potency score for hydroxyl group attached to beta-carbon of N-Nitroso function, below two structures can be considered in similar manner or as different.
R-O-CH2-CH2-N(R)-N=O
H-O-CH2-CH2-N(R)-N=O
The theoretic rationals for explaining the beta-hydroxy deactivating effect and the data supporting it is not similarly available for the ether built in the chain (in the sense to allow a wide variety in the R group), making the literal interpretation of CPCA beta-hydroxy (beta-OH) as depicted in the guidance the only relevant interpretation. Additionally, for beta-methyl activating feature, an NS OEG expert has clarified that this is obviously meant as a substituent, not as a part of the backbone. Equally the hydroxy should be understood as substituent. (Interpretation of CPCA should be done based on the scientific rationale behind implementing the factors, see also the CPCA exercise on N-nitroso-desmethyl-orphenadrine that is published by regulators.)
Although it is in my opinion not likely that an equivalent and general “beta-ether” deactivating factor can be developed any time soon: a deactivating/activating factor for an ether (in for example beta) (gamma oxygen in the chain) should regard what the “R” group is, cf. leaving group capacity of “RO” and its link with metabolisation risks to smaller NDSRI fragments (whereas typically only metabolisation data of the ether in the API is available (which goes often via the metabolisation to a primary amine first), whereas for the NDSRIs that can be significantly different).
The number of nitrosamines in the guided AI lists with an oxygen in the chain instead of a gamma- or delta-carbon does confirm the interest in understanding the mutagenicity of such NDSRIs.
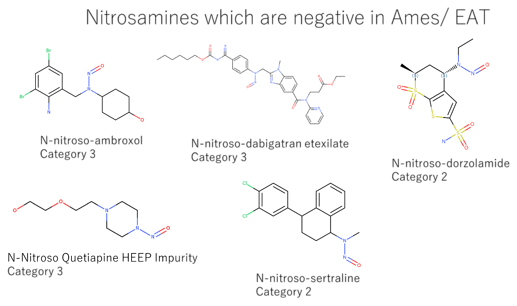
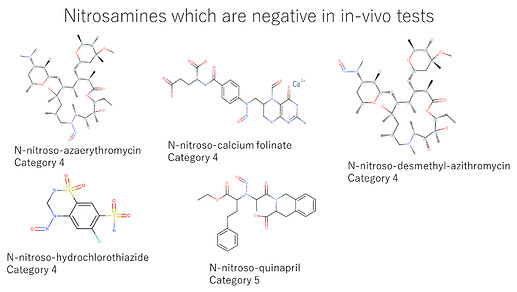
I want to focus on nitrosamines with negative in-vitro mutation tests and nitrosamines with negative in-vivo tests in EMA Q&A Appendix 1. Due to a lack of information, it was impossible to differentiate between the Ames test and the EAT. Nitrosamines in CPCA categories 2 and 3 showed negative in Ames/ EAT. On the other hand, nitrosamines in CPCA categories 4 and 5 showed negative in in-vivo tests. It means that those tests work well for de-risking.
The classification of CPCA in the AI list as of April 2024 is shown.
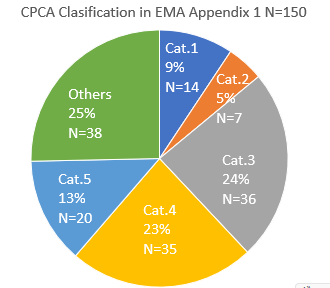
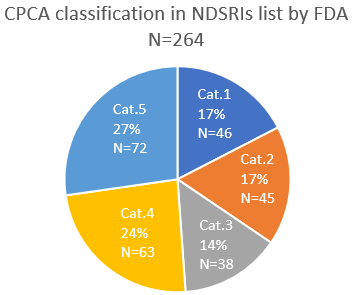
really interesting comparison Yosukemino, thanks a lot for sharing.
I have Hydrazide after oxidation it may form N-Nitroso amide which have No alpha carbon and (NH2-N=O ) is it mutagenic or not can you please conform and reply please