Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
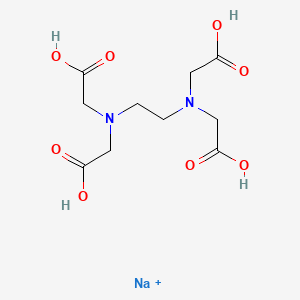
I know that disodium EDTA has a tertiary amine, do you consider that disodium EDTA can form nitrosamines?
Thanks in advance!
Laura Parra
Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
I know that disodium EDTA has a tertiary amine, do you consider that disodium EDTA can form nitrosamines?

Thanks in advance!
Laura Parra
The tertiary amino groups of EDTA is generally considered quite reactive; see the document below:
N-nitrosamine Impurities in Biological Medicinal Products
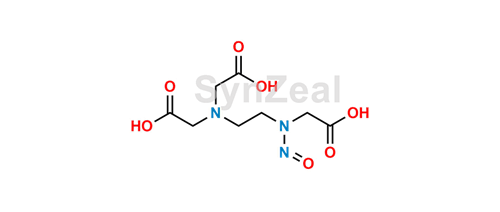
So, theoretically EDTA may be decarboxilated and nitrosated, forming
N-Nitroso Ethylene Diamino Triacetic Acid (CAS 862542-34-5)
Synonyms: Glycine, N-[2-[bis(carboxymethyl)amino]ethyl]-N-nitroso;
According to the last EMA guidelines, the carcinogenic potency should be category 4:
Count of hydrogen atoms on each α-carbon [2, 2] and corresponding α-Hydrogen Score is 1
Carboxylic acid group is found, Individual Deactivating Feature Score +3
Score: 4
Potency Category 4 : AI = 1500 ng/day
However, water for injections should not contain any nitrite or other oxidising impurity, so in my opinion the risk is negligible (of course the risk depends by the other excipients in the formulation).
The comment about EDTA being actively decarboxylated giving rise to a nitrosatable amine as found in the attachment seems to be an opinion rather than supported by actual data. We have looked at the molecule and would suggest that under normal conditions and use there is no possibility of forming such an amine from EDTA. Does anyone have actual data?
According to the ECHA, EDTA seems to include a small amount of ethylenediaminetriacetate.
https://echa.europa.eu/documents/10162/5ed7db13-e932-4999-8514-378ce88ca51f
True, but ECHA considers Edetic Acid (EDTA) in all industrial uses. Therefore, for most of them EDTA (and/or Disodium edetate) are technical grade, not pharmaceutical grade.
In both EP and USP monograph of Edetic Acid (I don’t have the JP monograph), the only impurity is the nitrilotriacetic acid (max 0.1% in EP and max 0.3% in USP).
In both EP and USP monograph of Disodium edetate, the only impurity is the nitrilotriacetic acid (max 0.1% in both).
If N-nitroso ethylendiamine triacetic acid will be present, I suppose that it should be easily detected by the EP (or USP) HPLC method.
Theoretically, EDTA could also form by dealkylative nitrosation an alternative derivative
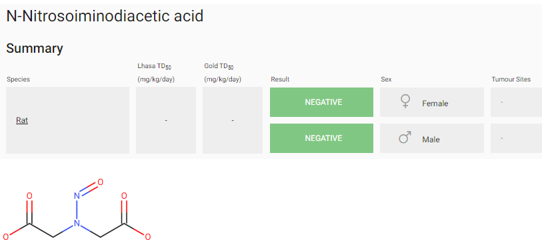
with test results included in LCDB.
These data may also support low/no risk for
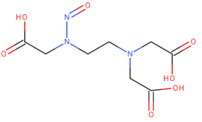
There is a (besides the above mentioned ones) reference to this in the following publication:
Formation of N‑Nitrosamine Drug Substance Related Impurities in Medicines: A Regulatory Perspective on Risk Factors and Mitigation Strategies (https://pubs.acs.org/doi/pdf/10.1021/acs.oprd.3c00153) alias “introduction of a nitrosatable amine in the mix”.
However, besides the theoretically introduction, I haven´t seen this case in real-life confirmatory testing yet. But this may be a future topic of “excipient quality grade” where dependent on grade/source a certain amount of nitrosatable amine is introduced in the finished product formula.
it can be easily analysed by HPLC-UV according to its limits.
Revisiting this. This was mentioned in Drug Substance and Drug Product Workflows for Quality Risk Management for the Presence of Nitrosamines in Medicines | Organic Process Research & Development as not forming. Has anyone tried an NAP test?