Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
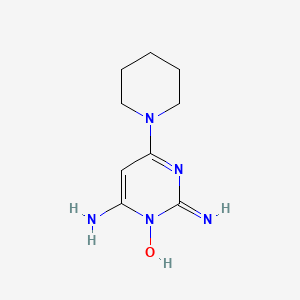
I know that minoxidil has a piperidine in its structure, do you consider that minoxidil can form nitrosamines?
Thanks in advance!
Laura Parra
Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
I know that minoxidil has a piperidine in its structure, do you consider that minoxidil can form nitrosamines?

Thanks in advance!
Laura Parra
Laura_Parra; The presence of piperidine, a secondary amine, in the raw material for Minoxidil production raises concerns about potential nitrosamine impurity formation. This could occur if nitro sating reagents are used during the process or if nitrous acid considered from the environment, as piperidine is susceptible to nitrosation due to its secondary amine nature. Also, piperidine is part drug substance structure and will generate during storage of Minoxidil.
Thank you very much for your appreciation. I was not sure if minoxidil would form nitrosamines, but I have more clarity with your explanation.
It seems that the reactivity of the minoxidil molecule itself toward nitrosating species is quite complex and interesting to analyze.
First, there is the piperidine fragment linked to the activated aromatic pyrimidine which may be more reactive than simple trialkylamines, see for ex. “A Consideration of the Extent That Tertiary Amines Can Form N-Nitroso Dialkylamines” (Ashworth et al., OPR&D, 2023) https://doi.org/10.1021/acs.oprd.3c00073
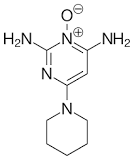
Next, the primary amine groups may act as scavengers for nitrosating species and even electrophilic substitution may occur at the aromatic ring, affording a C-N=O derivative.
Of course, the route of synthesis and the drug product composition and manufacturing process should be also considered.
In this case I would same this is an example of a C-nitrosation.
Aromatic C-nitrosation of a bioactive molecule. Nitrosation of minoxidil - PubMed (nih.gov)
It depends on the route of synthesis the availability of a secondary amine, some of them uses a piperidine molecule at the end of the manuf., others a substitued piperidine at the beggining. Depending on the purge capability of the process, piperidine traces may be expected up to 0,10% based on Ph. Eur. However, a competitive reaction should be in place because of the C-nitrosation and would reduce the availability of nitrite.