Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
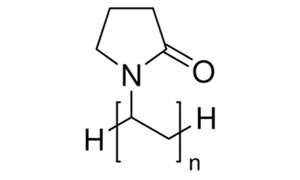
I know that polyvinylpyrrolidone has a pyrrolidine in its structure, do you consider that polyvinylpyrrolidone can form nitrosamines?
Thanks in advance!
Laura Parra
Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
I know that polyvinylpyrrolidone has a pyrrolidine in its structure, do you consider that polyvinylpyrrolidone can form nitrosamines?

Thanks in advance!
Laura Parra
In this case, I’d draw attention to two aspects rather than just the chemical structure itself.
Pyrrolidine and Amide Hydrolysis: When discussing pyrrolidine, it’s crucial to recognize that basic amide hydrolysis can create nitrosamine precursors. For instance, secondary amines can form through amide hydrolysis.
Nitrite Content: In specific instances, like with crospovidone, there may be trace amounts of nitrite present. These small nitrite traces can potentially react with vulnerable amines under favorable conditions, possibly leading to nitrosamine formation. Consequently, a thorough assessment of formulation components is essential to mitigate this risk.
Feel free to check these references for more information:
NDMA Formation Due to Active Ingredient Degradation and Nitrite Traces in Drug Product
Dear Lucas,
theoretically all you considerations are right. However, consider that:
Like other amides, pyrrolidones and caprolactams are susceptible to hydrolysis, particularly at elevated temperature and extremes of pH. The rates of hydrolysis at room temperature and neutral pH, however, are essentially undetectable
reference:
Introduction to Pyrrolidone and Caprolactam Chemistry
Dear @paliog , good point!
It is imperative that we engage in this discussion in the context of the Risk. We must endeavor to estimate the risk entailed by the recognized hazards while establishing a correlation between the probability of their occurrence and the gravity of resulting adverse consequences. For instance, in the case of amide solvents (such as DMF, N,N-dimethylacetamide, and NMP), which may potentially contain residual secondary amines stemming from their manufacturing process or undergo hydrolysis under specific operational conditions, a pertinent strategy involves refraining from utilizing amide solvents.