Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
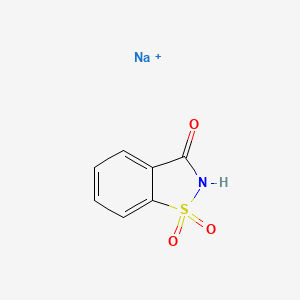
Would you consider that saccharin sodium could form nitrosamines?
Thanks in advance!
Laura Parra
Dear all,
I am working on a risk analysis of nitrosamines in some liquid products.
Would you consider that saccharin sodium could form nitrosamines?

Thanks in advance!
Laura Parra
Saccharin is a sulphonamide, not an amine. The nitrogen is not basic, because there are two strong EWG which delocalize the nitrogen pair. I never found reactions with nitrous acid or sodium nitrite.
A nitroso derivative of Saccharin has been reported after reaction with tert-butyl nitrite:
Artificial sugar saccharin and its derivatives: role as a catalyst
However, this compound has been supposed as intermediate; it was not isolated, nor characterized.
Also in another article, the same reaction has been reported and also in this case the intermediate (N-nitroso saccharin) was not isolated.
Arene diazonium saccharin intermediates: a greener and costeffective alternativ e alternative method for the pr e method for the preparation of ar ation of aryl iodide
I don’t think that this puculiar reaction should be considered as representative,
kind regards
What I could add here is that yes, the nitrosation of saccharin do not arrives to a nitrosamine that is the purpose of the guideline. In a common finished product manufacturing process I do not see the possibility of the nitrosation of this sulfonamide.
Also, is that for forming saccharin you can use a diazotation reaction (nitrosation of a primary amine). Therefore, it would be worth asking the manufacturer a synthesis diagram and if they use the di-azo reaction unless there is a nitrite scavenger somewhere in the synthesis, quite a bit of nitrite content per mg of sweetener could be present.