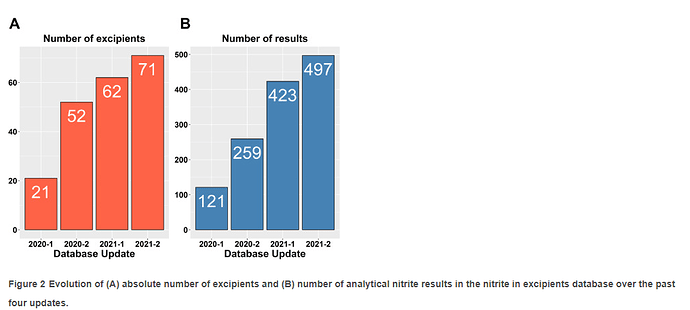
If you haven’t, I encourage you to read the recent publication " A Nitrite Excipient Database: A Useful Tool to Support N- Nitrosamine Risk Assessments for Drug Products" by Boetzel et al.
A fantastic update on the data-sharing consortium, maintained by Lhasa Limited. The goal of the initiative is to provide a common framework to contextualize and estimate the risk posed by the presence of nitrites to contribute to the formation of nitrosamines in drug products.
Key takes aways:
- Average nitrite content and batch to batch variance differ among excipients
- For solid dosage forms, the nitrite contribution is dominated by the highest formula % excipients, e.g., the fillers (diluents), which are typically used in larger proportion, and are characterized by low nitrite levels and low variability, leading to an average value of 1 µg/g nitrite in a typical formulation
- Substantial differences in average nitrite content in batches from different excipient vendors potentially reflecting differences in source materials or processing methods for excipient manufacturing
Our community members: @schlinjo1975 @giorgio @MarkWH @leonardo.allain are also contributors.