Hi, all.
It is an interesting thread. To understand the topic deeply, I add the figure of N-nitroso betahistine.
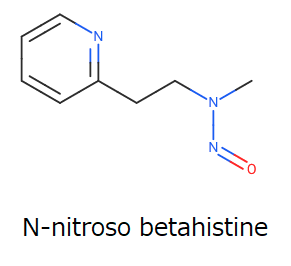
When I searched for analogs on LCDB substructure search with the following figure, an appropriate surrogate was not found, except for NMPEA.
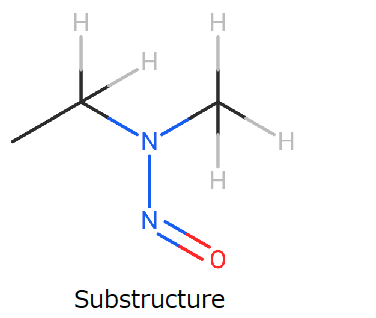
That is why I support @Diego_HM’s suggestion. If less than 10% of 8ng/day is confirmed, the betahistine will be free from nitrosamine confidently in most cases. It is a fantastic scenario!!
For other officially used surrogates, please see the following post.