In a recent public post, Moritz raised the following concern:
I am currently looking at a very unusual problem, and am wondering whether a group member might be able to help:
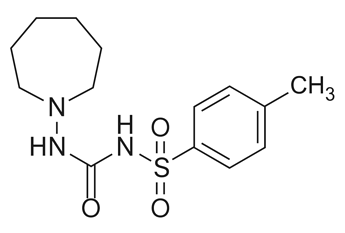
Some API seem to form N-nitrosamines upon oxidation (e.g. air) and without the presence of nitrosonium ions. For example Tolazamide was reported to form NHM upon oxidation.
Is anyone aware of any regulatory guidance on such problems or has anybody experience whether such an oxidation can be supressed effectively by antioxidants?
@AndyTeasdale shared:
Dear Moritz we are aware of this issue and agree that this has been observed now for several APIs. We have a group affiliated to EFPIA that are looking into this at present.
@fernandaw shared an interesting insight:
Tolezamide does contain a N-N bond and based on the paper by Lopez-Rodriguez (https://pubs.acs.org/doi/10.1021/acs.oprd.0c00323) oxidation of hydrazines/hydrazones is one route of formation of nitrosamines. I wonder if this might be similar to what happens for rifampicin and rifapentin? I would definitely ask for the chemists to help here