Hello All, anyone having experience working on NDSRIs of Duloxetine impurities like Nitroso Duloxetine Imp A, Nitroso Duloxetine Imp B, Nitroso Duloxetine Imp C, Nitroso Duloxetine Imp E and Nitroso Duloxetine Imp F.
Hello!
Can you be a little more specific about your problem so we can try to help you, perhaps through brainstorming?
Is your problem with methodology? Acceptable intake?
There is a topic by @Yosukemino about N-nitroso-duloxetine methodology: Simple and Practical Method for the Quantitative High-Sensitivity Analysis of N-Nitroso Duloxetine in Duloxetine Drug Products Utilizing LC-MS/MS
Hi, my name is Da-Kong, I am working on determining N-nitroso duloxetine (NDXT) in API with LCMSMS. I find different quantitation MRMs of NDXT were used in different references such as 344 (M+NH4)+ > 183, 183 (?) > 123 and 327 (M+H)+ > 183 . It could be affected by different mobile phase composition w/wo ammonium ion. Interestingly, we also find m/z 183 is the most abundant Q1 mass without the presence of ammonium ion. That is the same as what in the swissmedic’s mehtod and USP Product Information Sheet of N-Nitroso Duloxetine Solution. However, I have no idea how m/z 183 is produced. Is it reasonable from NDXT’s chemical structure? Should I use m/z 183 as the parent ion?
Hi chirag i am from formulation, so just in curiosity. you mean to say that Duloxetine N-imp (NDSRI) is having 5 different types or you are talking about routine process imps of duloxetine which named as Imp A B C…?
Thank you for your questions. Let’s address them step by step:
-
Yes, the use of ammonia-based buffers (such as ammonium formate, ammonium acetate, and ammonium hydroxide) can lead to the formation of ammonium adducts, specifically [M+NH4]+, rather than the protonated molecule.
-
Regarding the monitored MRMs, please note that the Swissmedic methodology relies on ion trap mass analyzers.
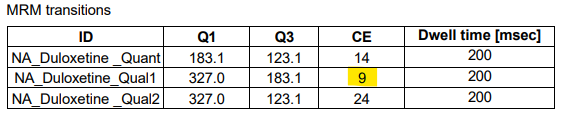
In this case, the authors of the methodology can “track” the origin of each fragment. They identified that from the precursor ion (m/z 327), the following product ions are formed: 327 > 183 (formed via remote hydrogen rearrangement) and 327 > 123.
Additionally, the m/z 183 can also lead to m/z 123, each with its respective collision energy. The fragment m/z 183, formed via remote hydrogen rearrangement, is indeed the most intense in the spectrum (as you described).
According to the Swissmedic methodology, this fragment requires the least collision energy (9 eV).
I hope this answers your questions. If you have any further inquiries, feel free to ask!
Lucas
Thanks for the clear MS explanation Lucas…
Thank you for the reply. My understanding is the AB Sciex QTRAP 5500 is still a triple quad LC/MS/MS with a linear ion trap (Q3). Generally, for MRM mode, the most abundant molecule ion will be choosed as the precursor ion (Q1), which can mostly represent the target analyte. We know the 327 m/z is the protonized NDXT [M+H]+, and it is more reasonable to represent NDXT when acidic mobile phase applied. But how come Swissmdedic method choose the 183 m/z as the precursor ion of quantitative MRM? Interestingly, which is the same as what we found.
In the previous message you kindly gave, it reminded me the 183 m/z may be produced by in-source fragmentation of molecule ion. However, we have tried to lower the cone voltage (10 V to 2 V) and the source temperature (500 C to 300 C), but the 327 m/z was still not coming.
Recently, we use the 183 m/z as the precursor ion for the further method validation work, and keep searching any possible interpretation. By the way, our LC/MS/MS is Waters, XEVO TQ-S micro. We may need more evidence to explain the in-source fragmentation is due to the different designs of ion sources of LC/MS/MS, for example, the distance from capillary to oriface.
Fueling back this post, as I have received couple inquiries about 327 vs 183 m/z
@lucas10mauriz @ingcortes @mayank.bhanti @Amit091086 @Frabaneda @Rizi
Thank Naiffer. Let me try to help out.
If you look at reported literature for Duloxetine, the most common MRM transition is 298→154 which corresponds to a loss of 144 (1-naphthol group). Similar fragmentation pattern is seen for N-Nitroso Duloxetine 327→183 (Loss of 144). Hence the fragment of 183 is correct. This further fragments to 123 when the optimum collision energy is applied.
When ammonium acetate/formate is used as the mobile phase 344 is prominently formed which is the ammonium adduct (probably because of the ether linkage).
However under acidic conditions 327 is expected to be the most prominent. However 183 is generally the most prominent ion indicating in source fragmentation as the collision energy required is quite less (as correctly pointed out by Lucas) and hence used in the Swissmedic method. This is certainly instrument dependent and you will have to optimize and decide the best transition that works for you. If you want to demonstrate that 183 is formed from 327, then you can further optimize your compound parameters or try precursor mode scan.
Alternatively you can attempt to quantify using the ammonium adduct. However quantification via the ammonium adduct can be tricky and hence I would recommend to use a deuterated internal standard (and use its corresponding ammonium adduct in the transitions) to provide more reliable results.