This week ANAMDED (National Regulatory Agency from Chile) announced the voluntary recall of all Atomoxetine DP due presence of NDSRIs
Announcement:
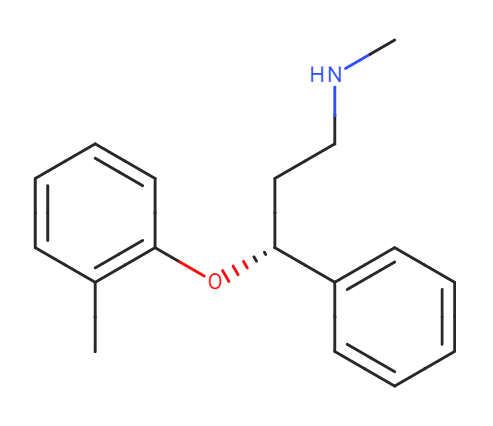
Smiles: CC1=C(C=CC=C1)O[C@H](CCNC)C2=CC=CC=C2
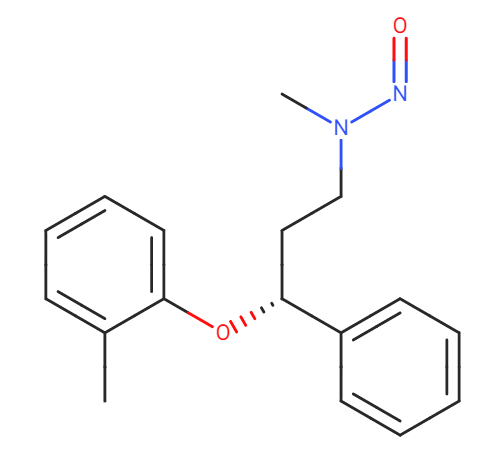
Smiles: CC1=CC=CC=C1O[C@@H](C2=CC=CC=C2)CCN(N=O)C
This week ANAMDED (National Regulatory Agency from Chile) announced the voluntary recall of all Atomoxetine DP due presence of NDSRIs
Announcement:
Smiles: CC1=C(C=CC=C1)O[C@H](CCNC)C2=CC=CC=C2

Smiles: CC1=CC=CC=C1O[C@@H](C2=CC=CC=C2)CCN(N=O)C

We can use NNK as point of departure for structure-activity-relationship (SAR) /read-across approach same as Nitroso Duloxetine and Nitroso Fluoxetine
Are you suggesting 4-(Methylnitrosoamino)-1-(3-pyridinyl)-1-butanone as surrogate?
SMILES: C1=CN=CC(=C1)C(CCCN(N=O)C)=O
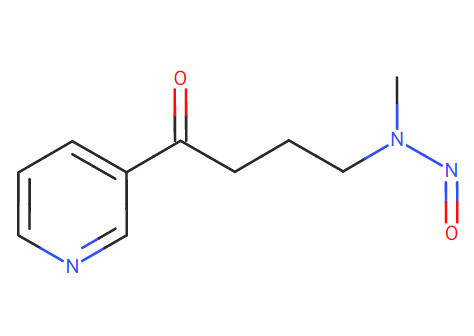
Lhasa Carcinogenicity database: Gold TD50 = 99.9 ng/day ?
Yes. EMA has published limit 100 ng/day for both Nitroso Duloxetine and Nitroso Fluoxetine based on NNK.
as per the current FDA NDSRI guidance recommendation, N-Nitro Atomoxetine falls under Category-1 with 26.5 ng/day.
Can somebody confirm this assessment?
Hi, @Hanimi.
FDA updated atomoxetine from 26.5ng/day to 100ng/day. Please check the following post.