@Nilesh, you are right, now many new Nitrosamines are been detected in the products.
Initially based on pre-evaluation of molecular structures of API, the probable Molecular Mass of new Nitrosamine impurity can be determined. Further, its presence in the product (using this Molecular Mass) can be confirmed during analysis using LCMS.
Later after confirmation, the new Nitrosamine impurity can be syntheses and the method can be validated.
Thanks a lot Sir… for your instant reply…
Sir, What is your opinion on below statement which is linked to last week question from my end
As per my understanding, AI limit for all Nitrosamine’s should be harmonized globally, as they are “Cohort of Concern” more carcinogenic/ mutagenic than Genotoxic Impurities.
@Nilesh It’s recognized that the testing of certain nitrosamine impurities requires the use of not just RS by also d-RS. USP runs a ‘Pharmaceutical Analytical Impurities’ program for these sorts of materials that are not intended for compendial use.
Perhaps you can reach out to one of my colleagues in USP for more information @FernandoUSP
@ Naiffer sir, Thank you very much for your reply…
@Nilesh, Currently harmonizing the AI limits is not possible. As I have mentioned, many new Nitrosamine impurities are been/shall be detected in the finish products due to presence of Nitrate/Nitrite contents in excipients used in formulations, hence to define the AI limits may take time.
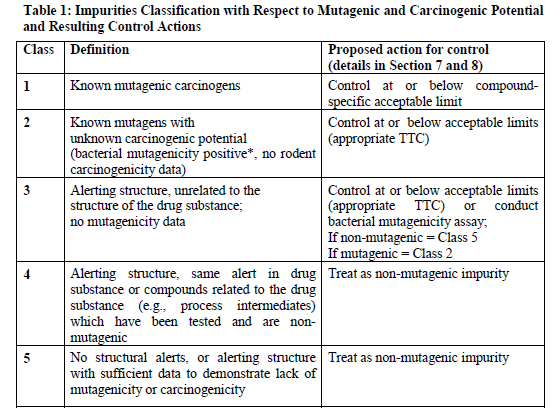
So to have an AI limits, ICH guideline M7(R1) is the option. Please check Section 6, Table 1 (as attached) and Section 7 & 8 of the guideline for the details, which may help in setting the AI limits.
Dear Jaideep sir & Naiffer sir…
Thank you for your reply & sorry for the delay to respond you…
Actually, my question & query as below, continuation with previous question…
As per “Health Canada guidance AI limit for 1-methyl-4-nitrosopiperazine (MNP) is 96.0 ng/day”, However as per EMA guidance is 26.5 ng/day.
and same for
As per FDA AI limit for N-Nitroso methylphenylamine (aniline), NMPA is 26.5ng/day, However as per EMA guidance is 34.3 ng/day.
Why two different Acceptable intake limit has been derived for MNP, by two different agency? and same for NMPA also.
As both are nitrosamine impurity, which is “Cohort of concern” & more carcinogenic than Genotoxic impurity.
Should it be harmonized globally, to that particular nitrosamine impurity, irrespective of market/regulatory agency?
Means “MNP” has same AI limit to both the market either 96.0ng/day or 26.5 ng/day and NMPA has same AI limit to both the market either 34.3ng/day or 26.5 ng/day.
Hope, I communicated my exact question to you and waiting for your Quick response till tomorrow morning as per Indian standard time (Date: 17-06-2022 morning).
I believe, you will clear my ambiguity…
Thanks
Nilesh
Requesting you Sir, kindly clear my ambiguity as earliest…
Thanks
Nilesh
@Nilesh The WHY of your question is a topic that continues to be controversial and in the center of a lot of regulatory-industry discussions. To me it’s not about the why, but about the WHAT we can do as a community to advance that conversation and build the necessary knowledge to justify the one or the other.
I suggest watching the recording of Dr. Nudelman (@conudel) and Dr. Cross (@kpcross) to dive deep into some of these regulatory approaches. Also curious to hear their opinions on the future of this particular challenge.
Thanks for your instant reply Sir…
Actually you are right sir… Instead of WHY, it should be WHAT could be the best approaches globally to harmonize the same…
I don’t want to pointing out any respected Regularity authorities bodies… It was just an ambiguity…as these are nitrosamine i.e. cohort of concern which impacted to the direct human DNA material which same all over globe.
Hi Team,
If there is insufficient carcinogenicity data available for a nitrosamine impurity, MAHs and applicants may apply the class-specific threshold of toxicological concern (TTC) of 18 ng/day for nitrosamine impurities as a default limit as per EMA & Health Canada guidance’s & 26.5 ng/day as per USFDA guidance’s.
May I know, HOW this Acceptable limit 18ng/day & 26.5ng/day as been decided/ fixed as default limit, as per respective HA.
Moreover, as per EMA & Health Canada guidance, N-nitrosonortriptyline is having AI 8ng/day which is less AI Limit & most potent nitrosamine.
If we think, it is worst case scenerio, then default option could be 8ng/day instead of 18ng/day.
Requesting all, could you please clarify above 2 queries…
Thanks
Nilesh
Hi, @Nilesh. You can find how EMA decided “18ng/ day” on the Assessment report of Nitrosamine. The corresponding part is as follows;
According to the document, 95% of nitrosamines are covered by this limit.
Thank you very much…
What about default option 26.5 ng/ day as per FDA?
FDA explains 26.5 ng/day as the AI for the most potent nitrosamines in the guidance.
Thank you let me check where it is mentioned & understand…
I will come back to you…
@Nilesh, sorry for delay in responding your query. Was busy with to other priorities.
I agree with you that the AI limits should be harmonized globally, irrespective of the regulatory agency. But as of now, we have to accept the different AI limits as per market/regulatory agency wise until it is harmonized.
Thank’s sir… for your reply…
No issue…
Dear All
Related to the topic I have one Query , Hope the experts around can help me,
Basically the straight forward question is about the limit of 4 nitrosamines, that can be possible in the process of one of the API (limit as below)
3.11 ppm for NEIPA (SMNI)
11,67 ppm for NDIPA (SMNI)
0.206 ppm for NDEA (SMNI)
0.778 ppm for NTTP (NDSRI)
Now what should be the control strategy , means what limit we need to follow, as I understood from the previous discussion, would we need to control the sum of all the nitrosamines should not be more than 0.206 (the most stringent one) ?
Dear Sudhir,
have you performed confirmatory testing and all these NAs are found at levels >10% LOQ?
Dear Eleni
thanks for replying, not yet, I would like to go for confirmatory testing, but I wondered about the LOQs for methods, In that case I think we can not only stick to LOQ just 10% of AI, but it should also be well below ?
Lets assume we have values >10% of AI, then what would be the strategy
again thanks
hi.
you could go for confirmatory testing for each nitrosamine to see whether each NA is actually found in your final product.
in case >10% then this NA imp should be included in final specs. In case more than one nitrosamines are identified in final product, then you should follow the principles as described under EMA Q&A 10 for Calculation of limit when more than one nitrosamine is identified in the same product
I trust you can also go through
Calculation of limit when more than one nitrosamine is identified - Limits of Nitrosamines - Nitrosamines Exchange
FDA Acceptable intake limit for multiple nitrosamines in one drug product -Calculation - Limits of Nitrosamines - Nitrosamines Exchange
Total impurities limit if more than one nitrosamine impurities are observed in product - Limits of Nitrosamines - Nitrosamines Exchange
Nitrosamine Calculation in Sita + Met Tablets - Limits of Nitrosamines - Nitrosamines Exchange