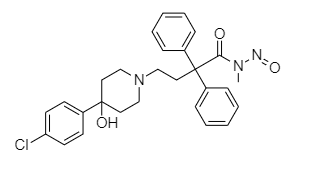
Smiles: O=C(N(C)N=O)C(C1=CC=CC=C1)(C2=CC=CC=C2)CCN3CCC(O)(C4=CC=C(Cl)C=C4)CC3
Hello experts, could someone guide the possibility of the formation N-nitroso Desmethyl fluoxetine? The application of the CPCA approach is not suitable for nitrosamines of this type. If it is possible, what would be the limit for Acceptable Intake (AI), and what method should be used for calculation? Appreciate your assistance in advance.
dear Sarada,
even i do not consider myself as an expert i would try to answer to your question.
If your API is the loperamide then, in order the N-nitrosodesmethylloperamide to be formed, demethylation following by nitrosation of the amide should be happened.
Nitrosation of amides is very difficult as the carbonyl grour delocalised the negative charge on nitrogen.
Overall, i would rather focus to defend the non-possibility of the formation of this nitrosamine.
If you are speaking for a final product i would also measure the pH of the product (if this was over 7 then even demethylation could be very difficult).
2 Likes
Thank you very much. It is helpful. @chrischar
1 Like
Hi Sarada.jena
Even though that would form it is a nitrosamide. They do not follow the same metabolic path as the nitrosamines, and therefore, they cannot be compared or treated as nitrosamines.
What I would flag here as possible NDSRI is:
4-(4-chlorophenyl)-1-nitrosopiperidin-4-ol
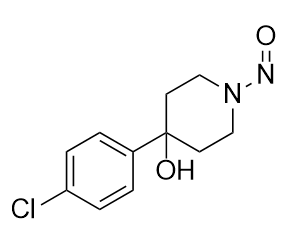
OC1(C2=CC=C(Cl)C=C2)CCN(N=O)CC1
That might form either via dealkylative nitrosation or direct nitrosation if this substructure is present as an impurity as vulnerable piperidine (P.S., I am not aware at the moment of the synthesis route nor known pharmacopeia impurities)
Regarding what you wrote in the message:
“Hello experts, could someone guide the possibility of the formation N-nitroso desmethyl fluoxetine?”
That is also possible with the above-mentioned explanation.
4 Likes
Thank you very much @Riccardo_Provenzani . It helped a lot.
1 Like

