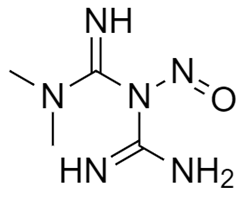
Dear team, I seek your insights and guidance regarding N-Nitroso Metformin. According to one of our API suppliers, the theoretical possibility of the NDSRI has been evaluated based on the API structure. However, the supplier faced challenges in synthesizing this impurity, deeming it a difficult task, and the CPCA approach was found to be inapplicable. This has left us in a state of confusion on how to address this issue in the finished product. While searching on Google and various impurity suppliers’ websites, we observed that this particular impurity is highlighted. Could anyone provide suggestions on how to calculate the AI (Acceptable Intake) for this impurity? Additionally, finding a structurally similar compound for read-across has proven elusive. Any assistance or recommendations would be greatly appreciated. SMILES: N=C(N(C)C)N(N=O)C(N)=N
IUPAC NAME: 1-carbamimidoyl-3,3-dimethyl-1-nitrosoguanidine
Impurity Structure:
API Structure:

I think that evaluating the AI would be useful only if you could obtain the nitroso derivative standard and perform testing.
While nitrosation of alkyl guanidines is possible, the products are nitrosamides (specifically, nitrosoguanidines) not nitrosamines, which do not undergo the same metabolic activation as N-nitrosamines (check for ex. “Nitrosamides - Should They Be Treated the Same as Nitrosamines?”, A. Srinivasan and C. Lambert, Pharm. Tech., 2022).
The chemistry of nitrosation may be complex yielding multiple products (e.g., Kinetics of Nitrosamide Formation from Alkylureas, N-Alkylurethans and Alkylguanidine, S. Mirvish, J. Nat. Cancer Inst., 1971) and the failed attempt to obtain it by the supplier is another good argument to rule out its presence.
The potential contamination of Metformin drug products with nitrosamines was extensively studied, including by several health agencies, mostly for NDMA, related to dimethylamine impurity coming from the manufacturing process of the API. There was never mentioned the potential presence of nitroso Metformin.
Many nitroso derivatives are offered by standard suppliers without actually being able to provide them and even if a compound can be obtained by a given synthesis route, direct nitrosation is not necessarily possible.
@mflorea Thank you greatly for your response and for taking the time to address this.
excellent response…very detailed in a few words and very well justified.
thanks a lot mflorea
Dear @Sarada.jena
Please ensure if N-nitroso metformin is completely absent in your product.
Even though it is not nitrosamine, if it is observed, it still qualifies for steps to control because they are direct mutagens and principles of ICHM7 applies.
Incase, if it synthesis is challenging- you may need to enter into dialogue with agency and pick different route of risk assessment and justification.
Nitrosometformin is not a nitrosamine but a N-nitrosobiguanide (a nitrosamide). So, if you are doing a nitrosamine risk assessment, please do not treat it the same way as you do the nitrosamines. I have addressed this topic in this forum long time back (Nitrosamides- Horse of a different color), even published a white paper which was shared with some FDA member more than a year back, and the agency has addressed this saying that they are not taking of nitrosamides. An API manufacturer should have Organic Chemists in their groups who should be able to tell the difference between nitrosamines and nitrosamides. Again, nitrosamides can be carcinogenic, but they are not part of the discussions and guidances that are currently going on. they are direct acting mutagens and a simple Ames can tell you if they are mutagenic. But at this time, agencies are focussing on nitrosamines and not the class of compounds you have drawn. The main nitrosamine risk of metformin is NDMA. And if you still want to make this nitrosamine in question, try to make it and isopropyl nitrite may be in xylen or other solvent where metformin dissolves did not work out, try Nitroso tetrafluoroborate as a nitrosating agent.
Please read my Pharm. Tech. paper, Nitrosamides–Should They Be Treated the Same as Nitrosamines?
Published on: October 15, 2022
Pharmaceutical Technology, Pharmaceutical Technology, Trends in Formulation, October 2022, Volume 2022 eBook, Issue 3, Pages: 42–50
An Ames Assay (direct) will tell one if this is mutagenic.
Also, to answer you quesiton on AI, as this could be a direct acting mutagen, I recommend an Ames Assay, if you want to address it now. If not, control it at 1.5 mcg/day like other mutagens in M7.
Thank you very much @ASrinivasan for the guidance.