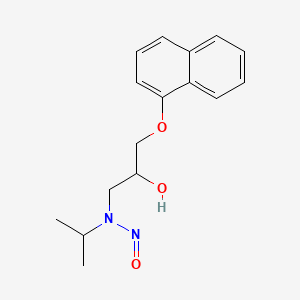
This week we learn from Health Canada about the presence of a nitrosamine impurity ( N -nitroso-propranolol)
Are you been affected? What do we know?
This week we learn from Health Canada about the presence of a nitrosamine impurity ( N -nitroso-propranolol)

Are you been affected? What do we know?
Hello, guys. Some specialist please tell us how this molecule is formed.
Naiffer,
can you please add some more info?
Thanks.
BR
Here is the official statement by Health Canada:
recall (propranolol hydrochloride) capsules due to a nitrosamine impurity - Canada.ca
Regarding the root cause, it could be several things. But, just to mention propanolol in solution has an interestingly low pH…
Hi, @Diego_HM.
Thank you for sharing the information. And I add the information of the excipients of these products.
The inactive ingredients contained in Inderal LA capsules are: cellulose, ethylcellulose, gelatin capsules, hypromellose, and titanium dioxide. In addition, Inderal LA 60 mg, 80 mg, and 120 mg capsules contain D&C Red No. 28 and FD&C Blue No. 1; Inderal LA 160 mg capsules contain FD&C Blue No. 1.
As we discussed in the lactose topic, excipients from natural origins have the risk of nitrite contamination at a trace amount. The pKa of this product is about 9.5, and as you pointed out, propranolol hydrochloride is acidic in solution. We should assess this product carefully.
Just to add additional details:
Despite the market withdrawal, Health Canada also announced that patients can maintain the use of the drug as prescribed, although it is necessary to consult a doctor about other treatment options.
Interestingly, studies described in the literature indicate that the compound N-nitroso-propanolol does not present mutagenic potential when evaluated in an ames test in several strains or mutagenesis assay in mammalian cells, and that thus, it would be unlikely that this structure was carcinogenic.
Last year @fernandaw presented at USP-Sindusfarma Nitrosamine Workshop a case study on Nitroso-Atenolol Nitrosamine Workshop USP / Sindusfarma / ANF ACFB -live discussion - #8 by Naiffer_Host You can watch the recording in the link (1:38:07).
On the other hand, when evaluated by in vivo micronucleus assays in rats, N-nitroso-propanolol was able to present a slight increase in micronucleated cells in the liver, but did not exert the same clastogenic effects in bone marrow and spleen.
These data make the evaluation of the mutagenic and carcinogenic potential of N-nitroso-propanolol controversial and complex.
@fernandaw / @chakravarti_suman / @kpcross / @David Do you have any additional insight from your assessment to share?
Yes, the "lol"s are coming. All the betablockers are beta-hydroxyamines and very prone to nitosation
Naiffer,
Can you provide references for the testing you mentioned?
Also see the 1991 free article by Robbiano in Cancer Research. Volume: 51, pp. 2273, 1991.
Kevin
Thanks Kevin. I am asking for the reference information to include here.
Adding your referenced paper here in the discussion: " Formation of the N -Nitroso Derivatives of Six β-Adrenergic-blocking Agents and Their Genotoxic Effects in Rat and Human Hepatocytes" by Robbiano et all.
Free access: Formation of the N-Nitroso Derivatives of Six β-Adrenergic-blocking Agents and Their Genotoxic Effects in Rat and Human Hepatocytes1 | Cancer Research | American Association for Cancer Research
Download: cr0510092273.pdf (1.4 MB)
With the information given this is one of the reasons the BoH do not want to completelly accept Ames test I understand, even if for most other cases it has been a very useful tool.
However, as with everything if there is no tox data, go for an in-vivo testing instead of an Ames test would take more time, and if everyone want to do it, it overcharge the available demand and do a bottle neck.
What I am wondering is what was the default AI used to determine the content was above the limit? 18 ng/day? Or an specific AI was developed.
Just to add to the discussion, Pfizer published recently an article related to AI in complex nitrosamines ( Dobo K.L. et al. (2022):). These proposed AIs were introduced last year in a conference on this topic by Patricia Parris.
The AI proposed in the article for betablockers is 440 ng/day.
Anyone is presenting this kind of AI to authorities?? Or 18ng/day by default?
Thanks for the information. I attach the link to the mentioned paper, it is a really good one.
Coming back to the topic, that is interesting for sure, because is not the same to have NA above the 18 ng/day limit than to 440 ng/day. This will need careful evaluation for al LOLs.
@hcamps thanks for sharing… below is the free access to " Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N -Nitrosamine Impurities" Dobo et all.
This paper also suggested relatively mild toxicity for NO-beta blockers
Toxicol Lett
. 1994 Sep;73(3):185-91.
doi: 10.1016/0378-4274(94)90057-4.
Quite interesting! Normally a Nitrosamine is formed by an amine reacting with a nitrosating agent (under nitrosating conditions, f.e. a certain pH). To identify the cause of this nitrosamine impurity, I recommend to look at all the reactants and the reaction conditions.
This is the structure of propranolol hydrochloride:
Propanolol has an acidic pH (2.8 -3.5), and therefore is able to react with a NO-. The NO- must be present in one of the reactants, as an impurity.
I’m not able to put this media file in, but here you could see the possible formation of a nitrosamine:
(File:Formation of n-nitrosamine.gif - Wikimedia Commons)
In conclusion the reactant, containing the nitrogen impurity should be identified, and made nitrogen free (or even replaced).
Thank you Tom , You explained well.
@Yosukemino
Thank you for the information of Drug product.
@ymleefn
Thank you for the information.