Has anyone seen official communication from Swiss Medic on their proposed changes to guidance?
You mean beyond the official 9 August 2023 letter that was resent together with the slides from the online event of 20 September 2023 on 10 October 2023? No, but this mail confirmed:
“The reporting template for the CPCA categories and the form for the declaration of consent (data sharing with other regulators) as well as the Q&A document (additional questions submitted via the chat during the event) will be sent separately later this month.”
I’ve seen a rather chaotic report from the online event, includes some interesting things, but couldn’t find anything official online in terms of updates to the guidance or forms to be used.
If they are coming later this month then I shall keep an eye out - thanks.
NDSRI CPCA reporting template.pdf (108.1 KB)
Swissmedic_Online Information Event_Nitrosamines_20.09.2023_Presentation.pdf (4.5 MB)
Swissmedic_Online Information Event on Nitrosamines_Q&A.pdf (490.5 KB)
Swissmedic has now made public most materials linked to the public online information event, which was building further on the 9 August 2023 letter to applicants:
- The CPCA reporting template
- The slides from the webinar
- The response to questions received during the webinar
Participants to the webinar have received those documents via e-mail this week. The template for the declaration of consent authorizing Swissmedic to share data with other agencies will still follow as soon as all translations are available (cf. discussion on readacross proposals etc. within NITWG; Swissmedic is one of the confirmed members of NITWG/NISG).
Thank you for sharing helpful information, @ccdw. I was so surprised to see it.
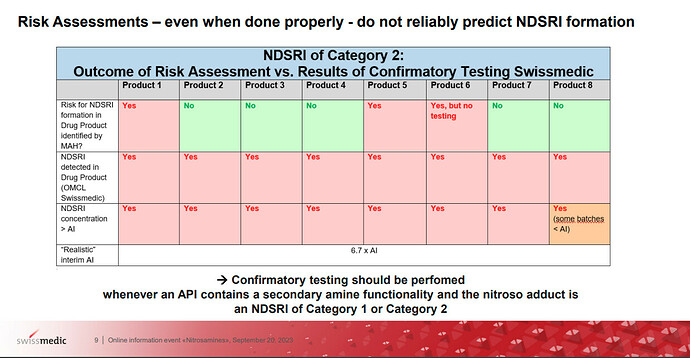
I found the slide describing the reality of risk assessment. It’s understandable. According to Q&A, Product 1 to 8 include the same API and they are provided from different manufacturers.
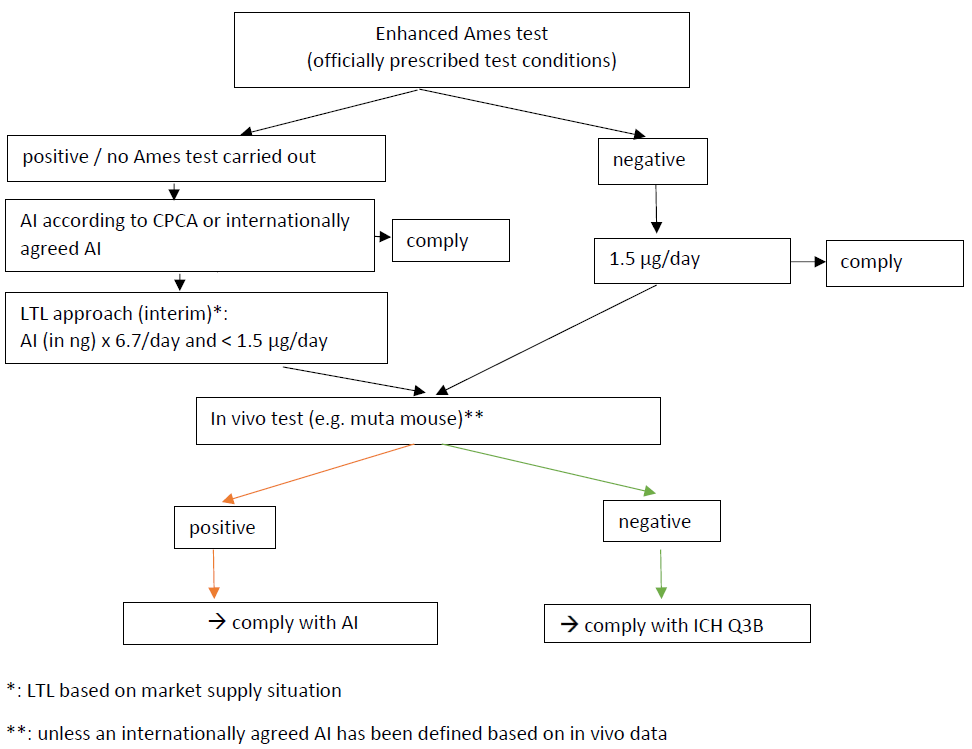
And swissmedic considers confirmatory testing is mandatory when nitrosamines from APIs with secondary amine moiety are in category 1 or 2 in CPCA. And Complete lists of products with the potential to form NDSRIs of CPCA Categories 1 and 2 are to be submitted to Swissmedic by 31.01. 2024. Now APIs with tertiary amine are out of scope with considering capacity. And impurity with secondary amine moiety is free from the request.
We should pay close attention to swissmedic activity.
This is what I had seen in the meeting minutes.
It is not possible to tell from this when those risk assessments were carried out though. Were they some of the very first ones done, where our knowledge was much less than it is now around NDSRIs and people/companies were focused on looking for nitrosamines from reused solvents etc.? Would the risk assessments be the same if the companies were to do them again now? Which leads to the question of how often companies are updating risks assessments in light of the increase in knowledge.
Remarkably indeed, there were no real indications that a data-driven risk assessment allows escaping the systematic testing obligation, also no indications that feeding toxicological data in the risk assessment to escape CPCA cat. 1-2 could allow no systematic testing.
Of note, Swissmedic also said a few times that their interpret FDA is de facto aligned with their approach:
“We discussed our strategy with EMA and FDA to attempt to get harmonization on the obligation for CPCA cat. 1-2 NDSRI testing. The FDA re-evaluation request is not so different in our opinion though (although giving more time we feel the position of FDA is quite similar).”
- Q: FDA does not request systematic testing linked to cat. 1-2 CPCA independent of risk assessment?
- A: That is correct, but we think our approach is reasonable based on the in house testing and experience gained by Swissmedic and the conclusion that for a big % the risk was missed. In many cases we already asked companies to test where we tested, but this exercise is still going on. Just risk assessment alone is not enough for cat. 1-2 CPCA.
Swissmedic September 2023.pdf (5.0 MB)
Here enclosed Swissmedic communication, including requirements, to this topic, which was directly sent to QPs: