Back in October 2020, we saw an increased concern about the potential presence of NDMA in Metformin. @trust_user_a @Nitrosamines_Analyzer @trust_user_c
“As of October 2020, EMA and the national competent authorities are asking marketing authorization holders for metformin-containing medicines to test their medicines before releasing them onto the market,” said EMA’s Committee on Human Medicinal Products (CHMP) in an update to its post-authorization nitrosamine impurity testing procedures”
As a Nitrosamine knowledge community, our center of attention lies in the understanding of how it happened and how to prevent it. I applaud the efforts of so many scientists in the community that pushes their organizations to share openly the lessons learned and the results of their research.
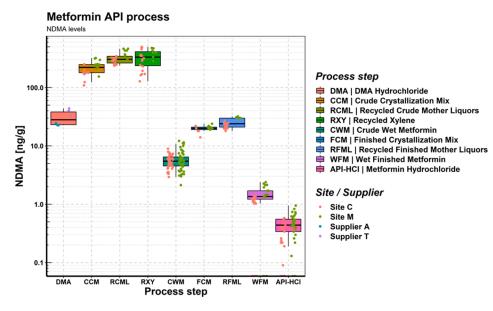
Joerg (@schlinjo1975) et all. just published ‘Avoiding N-Nitrosodimethylamine Formation in Metformin Pharmaceuticals by Limiting Dimethylamine and Nitrite’… They have performed an extensive analysis of NDMA concentrations along the API manufacturing process, and data mining exercise based on NDMA analysis of more than 2000 historical batches.
Please share you comments & questions here with Joerg.
Open access: https://www.sciencedirect.com/science/article/pii/S0378517322002952?via%3Dihub