I am sharing a couple pieces written by Andrew Collentro regarding Risk of Nitrosamines in Pharmaceutical Water. The original LinkedIn can be access using the links below:
Nitrosamines in Pharmaceutical Waters: Origins, Risk Mitigation, and Testing Requirements
Andrew Collentro
Pharmaceutical Water SME | Industry Consultant | Searcher
Nitrosamines are organic compounds, long known to be probable carcinogens, yet are found in low level concentrations in air, water, soil, and food. Both FDA and EMA have outlined a three step framework for mitigating or preventing the presence of nitrosamines in medicinal products which includes the following:
- Risk assessment
- Testing
- Risk Mitigation Measures
Water has been identified as a potential source of contamination of nitrosamines in pharmaceutical synthesis. FDA’s publication Control of Nitrosamine Impurities in Human Drugs Guidance for Industry Rev 1, FEB 2021 raises the following concern regarding API manufacturing:
“API manufacturers should be aware that potable water used in API manufacture may contain low levels of nitrite and even nitrosamines from environmental contamination. The existence of nitrites in processing water may lead to nitrosamine contamination in API manufacture. Therefore, to avoid unacceptable levels of nitrosamine impurities in APIs, API manufacturers should analyze nitrite and nitrosamine levels in water and use water that has been purified to remove unacceptable impurities.”
Sources of Contamination
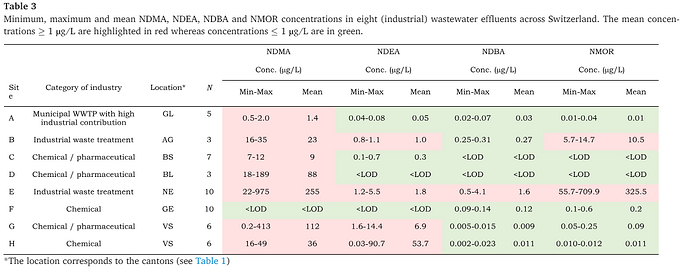
Nitrosamines are formed through a series of mechanisms but most generally from the reaction of amines with nitrites. Several forms of nitrosamines exist with the most common being N-nitrosodimethylamine (NDMA) which can be found in drinking waters treated with chlorine or chloramines. There are two most likely sources of nitrosamines contamination in pharmaceutical product waters. They may be present in the feed water to the treatment system (potable water) and not subsequently removed by a treatment process. Second, they may be unfavorably generated as a by-product of the treatment process itself when nitrogen containing compounds such as amines are present. The recent release of EMA’s Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products Rev 21DEC2022 outlines one potential source of nitrosamine contamination when ion-exchange technology is used as part of the water treatment process.
“ Reaction of amines leaching from quaternary ammonium anion exchange resins (e.g. used for purification steps) with nitrosating agents present in the liquid phase. A recent example of this was in the production of water for injections where residual chloramine used to disinfect incoming water reacted with dimethylamine leaching from the anion exchange resin used in the demineralisation step to form NDMA. In addition, disinfection procedures such as e.g. chlorination, chloro-amination and ozonisation can lead to significant N-nitrosamine generation as by-products in case vulnerable amines are present.”
Establishing a Nitrosamine Specification for Pharmaceutical Waters
It is the responsibility of the drug manufacturer to ensure that the product does not contain impurities that may alter the strength, identity, purity or efficacy of the drug product. This logic extends to pharmaceutical waters. In addition, any foreign impurities, even if their testing are not mandated by the pharmacopoeiae, are not permitted above acceptable limits. An acceptable limit is based on the product and risk to patient and may well be different for different drugs or classes of pharmaceutical preparations. Hence, acceptable limits of nitrosamines may vary for different grades of waters based on their intended purpose. A risk assessment should be used to to determine if nitrosamine detection is required for pharmaceutical waters, and if appropriate, the acceptable concentration limit, sampling location(s) and frequency
The EU trade organization recently commented on the presence of nitrosamines in WFI. EFPIA’s N-nitrosamine Impurities in Biological Medicinal Products, dated 11 November 2020 states the following:
Throughout this paper, reference is made to the negligible level of nitrite in the water for injection (WFI) used to manufacture and store biological medicinal products. When WFI is manufactured by distillation of Purified Water, it is expected that there would be essentially no nitrite or other known nitrosating agent present. Since the source Potable Water used to manufacture WFI can vary in nitrite/nitrate content [2], any subsequent Purified Water or WFI manufactured from Purified Water by methods other than distillation (e.g. reverse osmosis combined with ultrafiltration or deionisation), should be evaluated to assure that it too is essentially free of nitrosating agents.
The concentration of nitrite in Purified Water of WFI is expected to be far below 2×10-7 M (approximately 0.01 mg/L) as this level is typically seen in potable water.
This suggests that testing, to a maximum specification of 10 ppb, is required for the presence of nitrosating compounds in final product water when the WFI is generated by two specific methods; either by distillation when not fed from Purified Water, or when generated using non-distillation technologies.
Removal of Nitrosamines in Pharmaceutical Water Systems
Removal or reduction of nitrosamines to acceptable concentrations in pharmaceutical water systems can be accomplished by several methods or a combination of methods. Due to the light molecular weight of the compounds, reverse osmosis (RO) will likely reduce concentrations but may not be the most efficient process. Adsorption via actuated carbon is even less effective. High dose UV light and advanced oxidation techniques have been proven effective for both water and wastewater treatment applications.
Removal of nitrosamine precursors such as nitrite and nitrates prior to ozonation, and controlling the leaching of amines from ion-exchange resins are also effective techniques. The latter may be accomplished by avoiding stagnant DI tanks and periodic rinsing of anion or mixed bed DI systems when not in use.
Path Forward
Future guidance from industry organizations and regulatory agencies regarding the presence of nitrosamines in pharmaceutical water preparations is expected. Although a specific limit for these compounds may never be adopted for compendial waters, all drug manufacturers are responsible that water used in the manufacture of drug compounds or drug components contain no added substances, nor any foreign substances or impurities. Regardless of the source of contamination, it is likely that the presence of nitrosamines in pharmaceutical waters will be more rigorously scrutinized in the future.
Nitrosamine Testing Requirements for Pharmaceutical Waters
Andrew Collentro
While the topic of nitrosamines in drug products continues to be a widely discussed topic, there is still no guidance or mandated maximum allowable levels of nitrosamines, or their precursors, in pharmaceutical waters. Several groups and agencies have suggested that water is a potential source of nitrosamine contamination in drug products, but again, no specific limitations on these compounds have been proposed for water. We had recently discussed the sources and risks associated with the presence of nitrosamines in pharmaceutical waters here:
Nitrosamines in Pharmaceutical Waters: Origins, Risk Mitigation, and Testing Requirements
Since, additional guidance has been proposed regarding testing requirements for potable water. The draft copy of the “WHO good manufacturing practices considerations for the prevention and control of nitrosamine contamination in pharmaceutical products” (April 2024) suggests additional testing for nitrosamines and nitrosating agents in potable water as follows:
“Potable water is sometimes used in the production of materials such as excipients and APIs; or to clean equipment. Water may contain low levels of chloramine and or nitrites/nitrates, which are known to potentially react with secondary amines to form nitrosamine impurities, depending on specific conditions. The source, quality and purification of water may impact on the absence, presence or formation of nitrosamine impurities. For example, chlorination may contribute to the formation of nitrosamine impurities. Chloramine, nitrite/nitrate and nitrosamine levels in water should thus be determined.
Where required, water should be purified to remove unacceptable impurities before use.”
Potable water is often used in initial stages of drug manufacturing as well as preliminary cleaning and rinse steps for product contact surfaces. It is also the starting ingredient of any compendial water. Current testing requirements for any water used for pharmaceutical purposes ensures that the quality meets potable water standards, specifically the EPA National Primary Drinking Water Regulations or comparable potable water standard. This is outlined in USP monographs for pharmaceutical waters and is consistent with other international pharmacopoeiae.
Water used for production of finished pharmaceuticals, such as Purified Water and Water for Injection (WFI), must meet higher quality standards. Treatment of potable water used in pharmaceutical applications may include activated carbon, reverse osmosis, ion-exchange processes, as well as additional purification processes such as UV light or ozone. Compounded, these treatment processes reduce the levels of chloramines, nitrates and nitrites, and nitrosamines, to levels either undetectable by routine chemistry tests or in the parts per million or parts per billion ranges.
This proposed guidance from the WHO for nitrosamine testing in potable waters used in pharmaceutical applications is logical Potable water standards are not universal, chloramination is a common microbial control technique used by municipalities in the U.S., and naturally occurring nitrogen compounds can vary be present in feed waters. However, the suggested testing may not extend to compendial waters. Treated to meet standards and levels for other critical quality attributes, the concentrations of these compounds are likely within acceptable limits. A risk assessment can be used to determine if additional sampling or monitoring is required.
Risk Assessment considerations when developing test plans for nitrosamines and their precursors may include the following:
- Presence of chlorine and chloramines - These residual disinfectants found in feed waters are nitrosating agents that can react with amines to form nitrosamine compounds.
- Nitrite / nitrate levels - The presence of these compounds can lead to formation of nitrosamines as part of the water treatment process or when present in the product water, may lead to formation of nitrosamine impurities during drug synthesis
- Purity level of water or purification techniques used - The more the water is purified to remove other organic and inorganic impurities, the lower the expected concentrations or nitrosating agents and nitrosamine precursors should be.
- Anion exchange resin used as a treatment process - The use of ion-exchange resins as part of the treatment process, may actually increase the concentration of amines. In particular, strong base anion resins, which may elute quaternary amines, can lead to nitrosamine formation.
- Ozonated Systems - The presence of nitrogen compounds, especially nitrite and nitrate, may lead to nitrosamine formation.
- Water contribution to or presence in the final product - The amount of water used in product formulation, or the water activity for non-sterile products, should also be considered. In some instances, such as when the water is only used for cleaning and sanitization, then the risk of contribution of nitrosamines in the final product may be minimal. Both FDA and EMA have provided guidance on daily allowable intake (AI) levels for nitrosamine impurities in drug substances. A risk assessment should include an evaluation of how the water is actually used in the drug manufacturing process.
- History of high nitrate levels - Most Purified Water and WFI sampling programs have historically included testing for nitrates as required by the European Pharmacopoeia (EP). Although this testing requirement was recently removed from the EP water monographs, if high levels of nitrates have been observed, additional testing for these compounds and nitrosamines would be warranted.
A definitive specification for nitrosamine levels in Purified Water and WFI may never be promulgated. Although AIs for specific nitrosamine compounds in finished pharmaceuticals are now being introduced, maximum contaminant levels for these impurities in pharmaceutical waters are not expected. Regardless, by its nature, water remains a potential source of nitrosamine contamination and some level of testing is warranted.