Anyone having idea about AI limit of Nitroso Flecainide?
What could be the AI limit as per QSAR and read across approch?
Hi,
My first impression with the API structure would be to take N-nitrosopiperidine 1300 ng/day as a starting point as this nitroso compund is now in the oficial guidelines. However, I see two heavy electron withdrawal groups in the halogens (tri-fluoro groups), steric hindrance and so on that would mean a higher limit.
It should be checked if there is a more closely nitrosamine analog in Lhasa carcinogenic database with robust data for example.
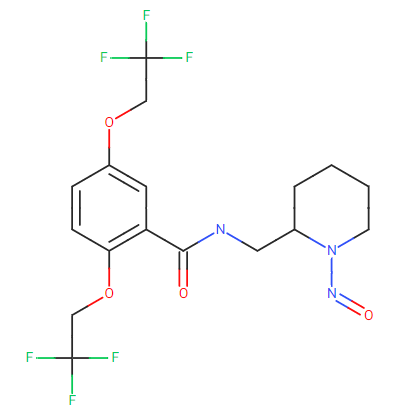
In any case, I know is not the question, but I would also wonder, althought to a less extent where does that piperidine came from and if it is expected in the FP. Impurity B in the Ph. Eur. gives some hints into it.
Anyways, I hope this helps.
Hi Diego_HM
There are two secondary nitrogen present in Flecainide.
Why we more focus on N-nitrosopiperidine.
Is it not possible to form nitrosamine by amide containing secondary nitrogen.
Please share if you have any article for same.
Hi Nitish, for that amine which is outside ring, having carbonyl group attached to it so it’s difficult to available for nitrotation. I am not having any article but you can opt for synthesis of the same in different types of reactions and generate a scientific justification based on the surrounding environment around that amine.
Hi, @Nitish.Sharma.
In EFPIA DP Workflow, amides are considered of lower reactivity and out of scope for DP because of the electron-withdrawing properties of the carbonyl group. And the reference of foot note3 (Opinion on Nitrosamines and Secondary Amines in Cosmetic Products) explains amides’ reactivity. Powerful nitrosating agents such as fuming nitric acid, N2O4, and NOBF4 are required to convert secondary amides into their N-nitroso derivatives, according to this. This is why you can focus on the secondary amine of Flecainide, I think.
Opinion on Nitrosamines and Secondary Amines in Cosmetic Products
P12
I want to add the link to the excellent topic with a toxicological insight for N-nitrosamide posted by @ASrinivasan. It’s marvelous!!
Dear Yosukemino San,
my PhD advisor Dr. Loepkky is repeatedly mentioned in the EMA document you mentioned. So it was good to see that people are paying attention to this. Sadly food and cosmetics took care of nitrosamies long time back, so did the rubber and metal cutting industry. Both, pharmaceutical industry and regulators shoved this under the rug and pretented that it did not exist. And now that it is all out, people are reinventing the wheel, sadly, it is a square wheel.
Hi, @ASrinivasan,
Thank you for sharing this helpful information. Yes, you are right. We should clarify the fatal nitrosamine precisely, but it will take time. As EMA Q&A is doing, repeated regulations update is essential depending on acquired knowledge. And I respect experts’ dedication to these problematic issues. This community helps understand nitrosamines’ features well. It’s fantastic!!
@ASrinivasan and @Yosukemino for this knowledge sharing, nitrosamide are difficult to generate by synthesis also and having lot of stability issue , so definitely based on this we can justify their absence in our finish product also.
Chirag, with two electronegative groups attached to each other, the nitrosoamides (when the amide has a primary amine) are quite unstable. Now, you may also be able to use this justification, by trying bench top experiments and showing that these do not easily thrive when heated or treated with acid or base.
