@trust_user_a @Nitrosamines_Analyzer @trust_user_c @trust_user_d
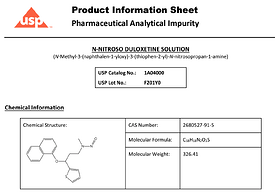
NDSRI are back in the news radar… This time Nitroso-Duloxetine
Source: ABC News (https://abcnews.go.com/GMA/Wellness/thousands-bottles-popular-antidepressant-recalled-3-things/story?id=115061455)
Thousands of bottles of popular antidepressant recalled: 3 things to know
Over 7,100 bottles of duloxetine are part of the recall, according to the FDA.
Thousands of bottles of a popular antidepressant medication are being recalled due to the presence of what the National Library of Medicine describes as a toxic chemical, according to a notice from the U.S. Food and Drug Administration.The recall involves the medication duloxetine, which is sold under the brand name Cymbalta, according to the FDA’s notice of the voluntary recall, which began Oct. 10.
More details
Duloxetine is part of a class of drugs known as SNRIs, or selective serotonin/norepinephrine reuptake inhibitors, that are used to treat anxiety, depression and other mood disorders, according to the FDA.
Here are three things for consumers to know about the recall.
1. How do I know if my medication is impacted by the recall?
The recall involves 7,101 bottles of duloxetine delayed-release capsules distributed nationwide within the United States, according to the FDA.
The recalled capsules are 20mg in strength, and sold in 500-count bottles.The lot number for the recalled capsules is 220128, with an expiration date of December 2024, according to the FDA notice. The recalled capsules are manufactured by Towa Pharmaceutical Europe.
In an emailed statement, Towa Pharmaceutical Europe referred ABC News to the FDA’s website on nitrosamine impurities in medications. N-nitroso duloxetine is a type of nitrosamine.
“With respect to nitrosamine impurities in drugs, FDA continues to say that ‘patients taking prescription medications with potential nitrosamine impurities should not stop taking their medications. Patients should talk to their health care professionals about concerns and other treatment options,’” Towa Pharmaceutical Europe said in a statement. “FDA has also said it is ‘working to determine the source of these impurities and will keep the public informed.’”
2. What is the potentially toxic chemical that sparked the recall?
The recalled duloxetine capsules were found to contain a higher level of N-nitroso-duloxetine than is permitted, according to the recall notice.
N-nitroso duloxetine is a chemical compound that can be toxic if swallowed in elevated concentrations and is suspected of potentially causing cancer, according to the U.S. National Library of Medicine.
The FDA designated the voluntary recall of duloxetine bottles as a Class II recall. The agency defines that as “a situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
I am including a summary of the community’s discussion on limits, analytical methods, mitigation, and standards in the post below. Click for more.